Roche has filed for European Union approval of the test and plans shortly to do the same with US health authorities, CEO Severin Schwan told the Swiss German SonntagsZeitung newspaper.
"We hope that they will grant us an accelerated authorization procedure," Schwan said.
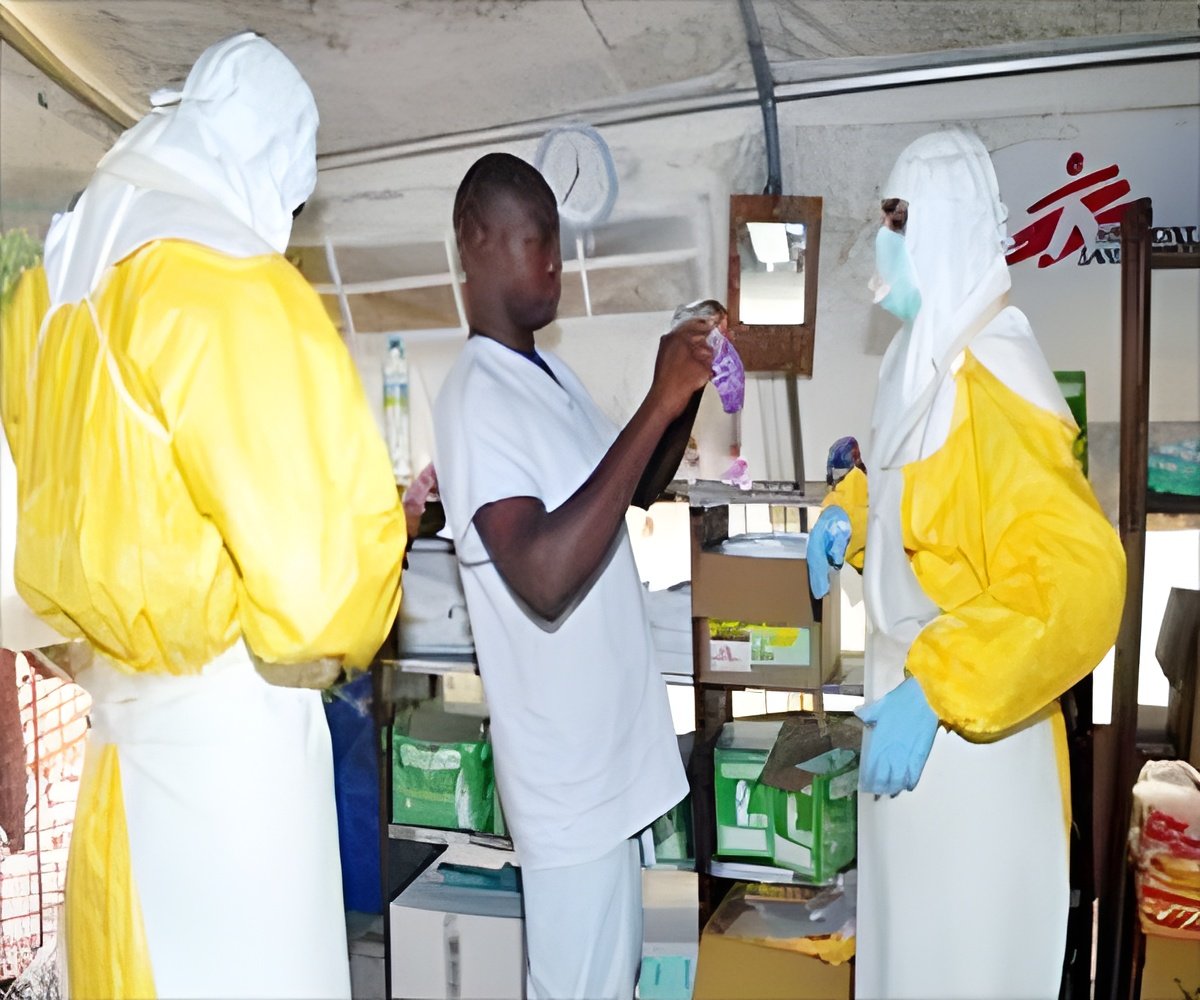
More than 10,000 people have contracted the Ebola virus, according to the latest World Health Organization figures, mainly in the west African countries of Liberia, Sierra Leone and Guinea.
More than 4,900 people have died of the hemorrhagic virus, according to the figures from October 23.
Source-AFP