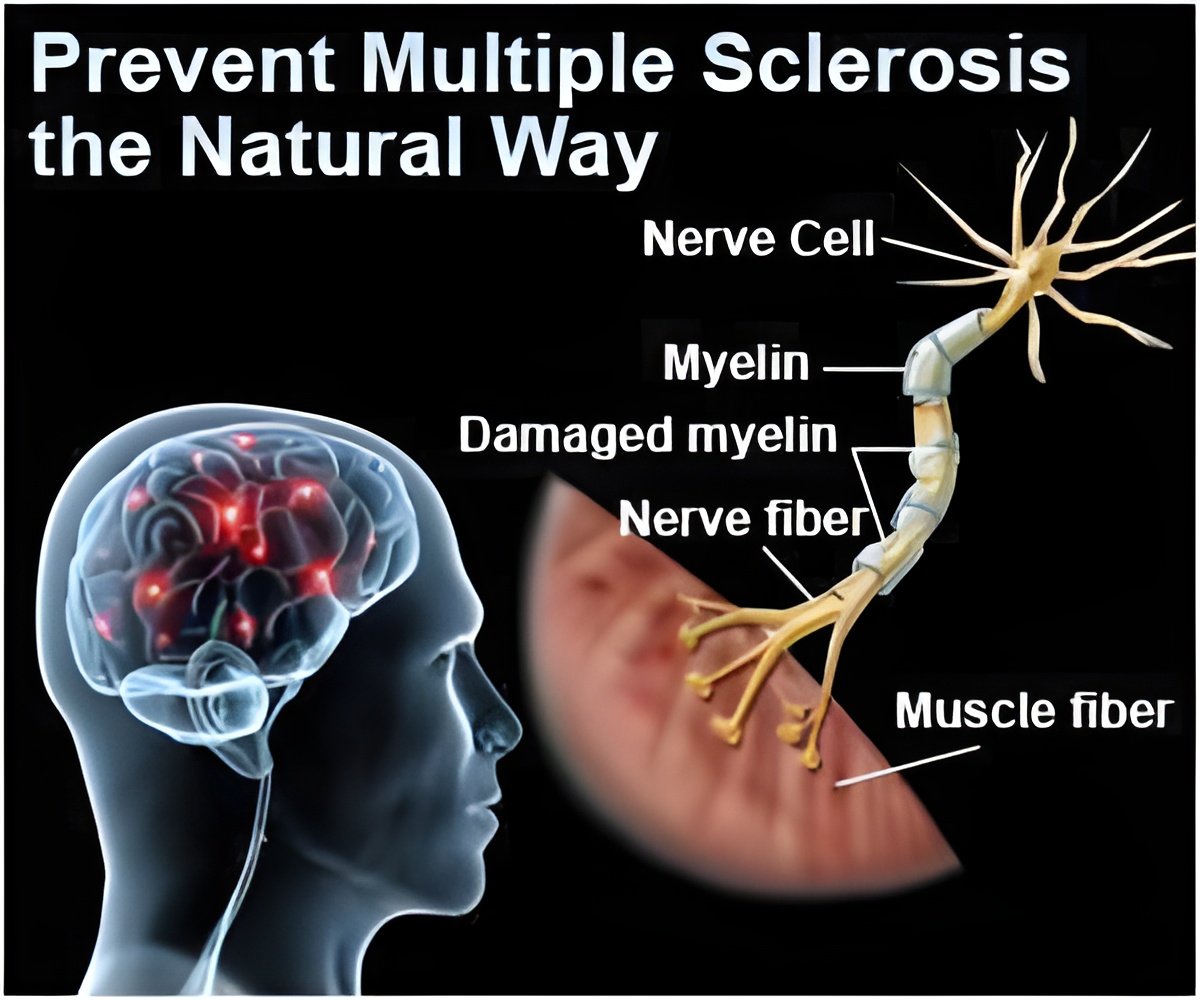
MedDay, a biotechnology company focused on the treatment of nervous system disorders, will present results of its pivotal clinical trial at The American Academy of Neurology (AAN) annual meeting in Washington DC, on April 24.
The trail investigates the efficacy and safety of MD1003 drug in the treatment of primary and secondary progressive multiple sclerosis (MS), a major area of unmet medical need.
MS-SPI is a randomized, double-blind, multi-center, placebo-controlled trial of MD1003, 300 mg/day, in patients with progressive MS.
A total of 154 patients with a baseline EDSS (Expanded Disability Status Scale) score of between 4.5 and 7 were enrolled from 16 MS reference centers across France. Treatment duration was one year.
Source-Medindia