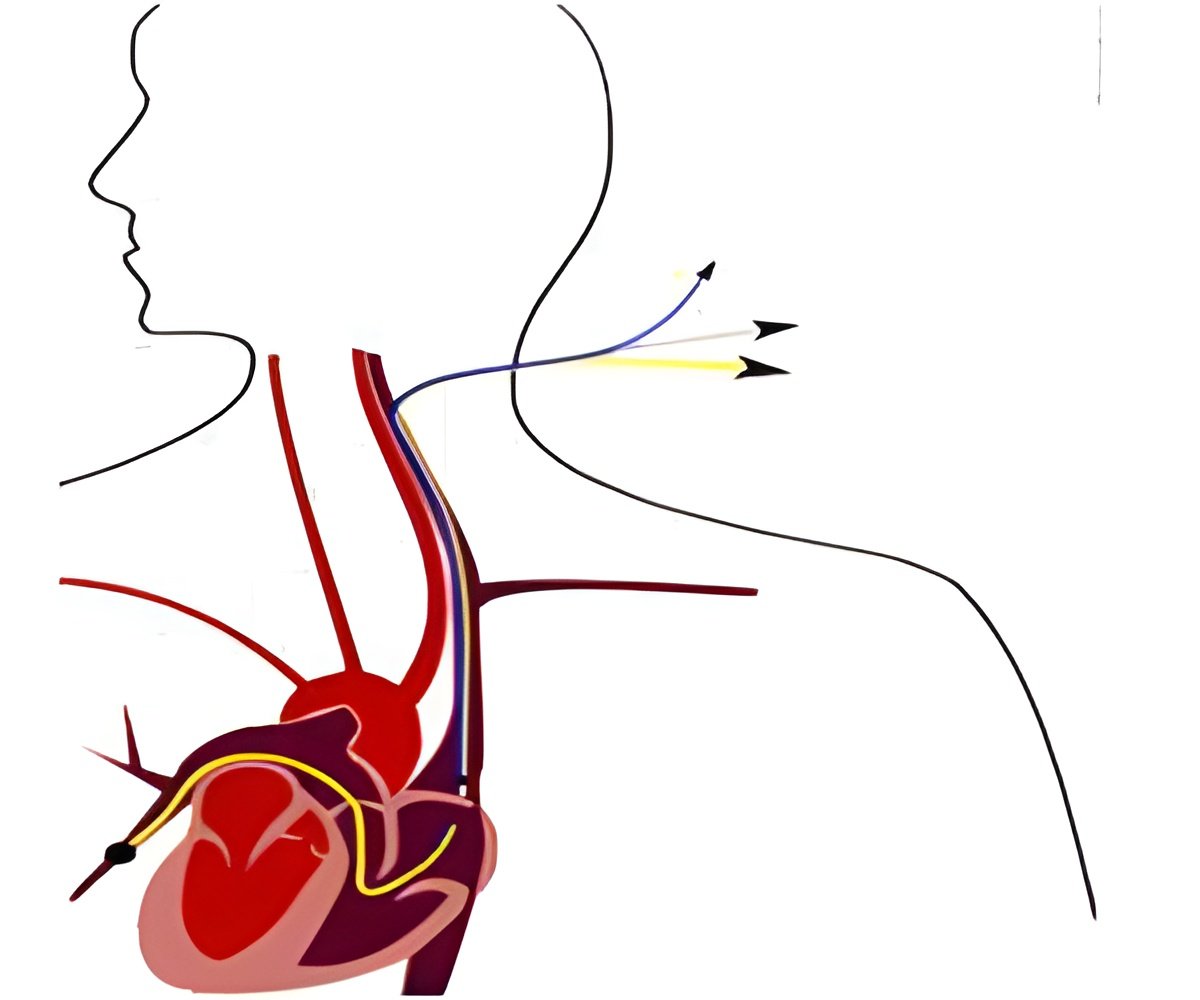
‘Features of ER-REBOA include its small 7 french scale size, which precludes the need for extra surgical repair at the access site. The catheter doesn''t require multiple wire exchanges. It also has a soft, atraumatic tip and offers simultaneous arterial pressure monitoring.’
Tweet it Now
"We are proud to be the first to market with a balloon occlusion catheter designed specifically for this community. They asked for the unique combination of features found on the ER-REBOA catheter, and we look forward to getting it to them," said David A. Spencer, CEO of Pryor Medical Devices.Features of ER-REBOA include its small 7 French scale size, which precludes the need for extra surgical repair at the access site. The catheter doesn't require multiple wire exchanges. Most importantly, it also has a soft, atraumatic tip and offers simultaneous arterial pressure monitoring.
The medical device company has scheduled first delivery of its catheters for January 1, 2016.
Source-Medindia


![Pulmonary Arterial Hypertension [PAH] - Symptoms & Signs - Causes - Diagnosis - Treatment Pulmonary Arterial Hypertension [PAH] - Symptoms & Signs - Causes - Diagnosis - Treatment](https://www.medindia.net/images/common/patientinfo/120_100/pulmonary-arterial-hypertension-pah.jpg)



