‘An investigational cell-based therapy for retinitis pigmentosa has demonstrated a favorable safety and tolerability profile in an ongoing Phase I/II clinical trial.’
Tweet it Now
Funded by the California Institute for Regenerative Medicine (CIRM), the trial has successfully undergone four reviews by the Data Safety Monitoring Board (DSMB). The first patient enrolled in the study, in which allogeneic human retinal progenitor cells were injected into one eye, has now been followed clinically for one year post-treatment. Interim (six to 12 month) safety results from this patient and the eight subsequent trial participants enrolled in 2015 are encouraging. "We are pleased with the results," said jCyte co-founder Henry Klassen, an associate professor of ophthalmology at UCI and a member of the Sue & Bill Gross Stem Cell Research Center, where much of the pre-clinical work took place. "This is an important milestone in our effort to treat these patients."
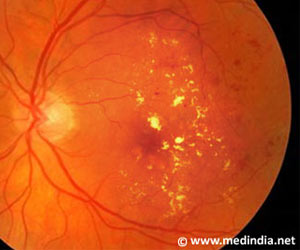
The cell-based approach taken by jCyte is intended to rescue sick and dying retinal photoreceptor cells (rods, cones) in the diseased retina. Nonclinical studies have shown that transplanted retinal progenitor cells tend not to provoke an immunological rejection response, even when sourced from unrelated donors. Because of this, patients in the study are not immunosuppressed, as is often done in traditional organ transplantation. In addition, the procedure is relatively simple to perform and can be completed in an office setting.
The ongoing Phase I/IIa safety trial is being conducted at the Gavin Herbert Eye Institute at UCI and at Retina Vitreous Associates in Los Angeles.
Based on the interim results from the current trial, further clinical studies are being planned.
Advertisement