Implants termed "unclassified" have had no evidence submitted by the manufacturers, while "pre-entry" products have less than three years of evidence. Yet many are widely available for implantation by an orthopaedic surgeon.
The team then reviewed the medical literature to establish the level of evidence available for these implants.
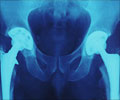
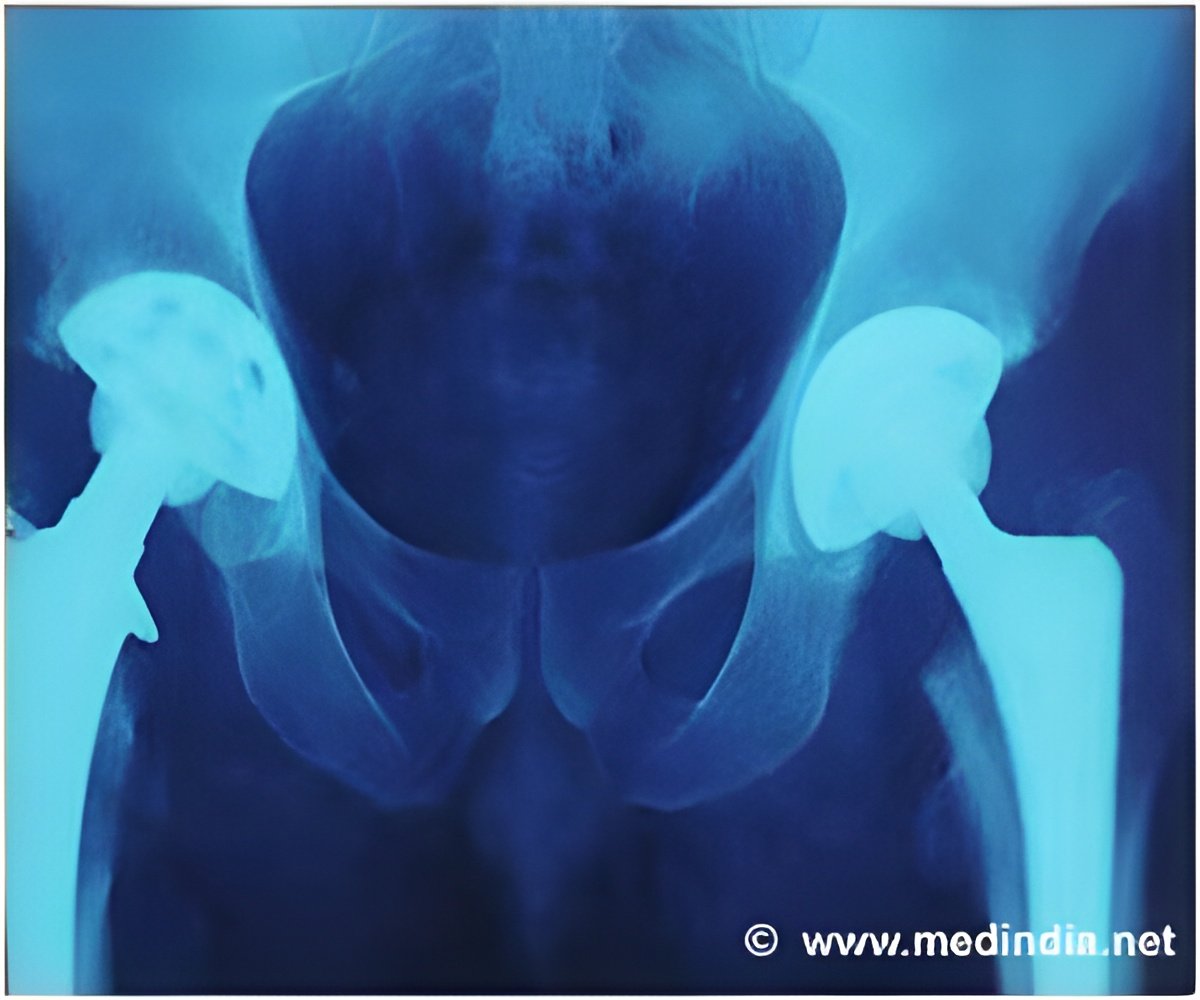
The review showed that 10,402 (7.6%) of the 136,593 components used in primary hip replacements in 2011 were implanted without readily identifiable evidence of clinical effectiveness. These comprised 157 cemented stems (0·5% of those implanted), 936 uncemented stems (2.8%), 1,732 cemented cups (7.1%), and 7,577 uncemented cups (17.1%).
This is of great concern, say the authors, "particularly in light of the widespread publicity surrounding recent safety problems with regard to some resurfacing and other large diameter metal-on-metal joint replacements."This is also likely to be an underestimation of the true problem, "as much of the evidence that does exist for the other unrated prostheses is of low quality or relates to short-term outcomes only," they add.
They suspect that the paucity of good quality evidence in this area may be related to the rapid expansion in the number of devices introduced onto the market during the past two decades, as demand for hip replacement surgery increases worldwide, and the difficulty of conducting high quality randomised controlled trials with orthopaedic implants.
Advertisement
In an accompanying editorial, researchers at Harvard Medical School argue that "the ability of manufacturers to promote devices or drugs that are authorized by regulators for widespread use but that do not have rigorous pre-approval data should be restricted."
Advertisement
Source-Eurekalert