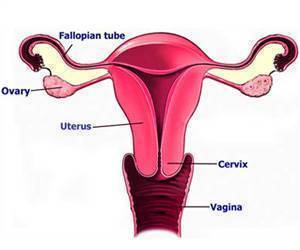
‘A new low-dose vaginal estrogen capsule may help relieve symptoms of menopausal vulvar and vaginal atrophy, including dyspareunia (pain during sex).’
Tweet it Now
"This study provides a new easy-to-use option for vulvar and vaginal atrophy, for which only about 7% of women are currently treated with a prescription product. Health care providers and their patients may soon have an additional safe and effective product for a very untreated condition," said study consultant and lead author Ginger D. Constantine, president and CEO of EndoRheum Consultants, LLC, in Malvern, Pennsylvania. TX-004HR, containing the estrogen 17β-estradiol, is currently an investigational drug for use in clinical trials and is not yet available to the general public.
TherapeuticsMD, the manufacturer of TX-004HR, conducted the double-blind, randomized, phase 3 REJOICE clinical trial comparing 3 doses (4, 10, and 25 micrograms) of TX-004HR with placebo in 764 postmenopausal women aged 40 through 75 in 105 medical centers in the United States and Canada.
The women in the study received either vaginal softgel capsules containing one of the three doses of TX-004HR or placebo, once daily for two weeks, then twice weekly for 10 weeks.
Within two weeks, at all doses, vaginal cells and vaginal pH significantly improved, compared with placebo. Superficial and parabasal vaginal cell improvement was found at baseline and at two, six, eight and 12 weeks, and at every time point the return to premenopausal cell ratios was significant. Vaginal pH returned to premenopausal levels as well. Dyspareunia, vaginal dryness and irritation, significantly improved.
TX-004HR did not, on average, increase blood levels of estradiol outside the normal postmenopausal range.
The treatment was well tolerated. No treatment-related serious adverse events were reported, and no clinically significant differences in any adverse events or treatment-related serious adverse events were found between TX-004HR and placebo.
TherapeuticsMD plans to use the results of this study in its new drug application for approval of TX004HR to the FDA that will be submitted during the first half of 2016.
Source-Newswise