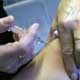
The British Government has written to senior neurologists to warn them that the new swine flu vaccine may trigger a deadly brain disorder called Guillain-Barre syndrome.
Medical experts have been asked to look out for cases of Guillain-Barre syndrome, which can paralyse its victims, once the national vaccination programme begins.In its letter, the Health Protection Agency refers to the use of a swine flu vaccine in the US in 1976, when 25 people died from the syndrome, while just one died from flu.
Concerns have already been raised that the new vaccine has not been sufficiently tested, and its effects, especially on children, are unknown.
About 13 million people will receive the jabs during the first wave of the programme, expected to begin in October.
The Health Protection Agency has asked neurologists to monitor closely any cases of GBS as the vaccine is rolled out.
A spokesman for the agency said that enhanced surveillance was "routine".
Advertisement
"Establishing enhanced surveillance on the syndrome has always been part of our pandemic plan because there is an increased risk of this disease after a flu-like illness," he added.
Advertisement
Source-ANI
SRM