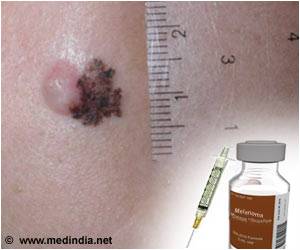
"Keytruda is the first approved drug that blocks a cellular pathway known as PD-1, which restricts the body's immune system from attacking melanoma cells," the FDA said in a statement.
"Keytruda is intended for use following treatment with ipilimumab, a type of immunotherapy."
More than 76,000 Americans are diagnosed with melanoma each year and nearly 10,000 die, the agency said.
The Melanoma Research Alliance described the approval as a "major breakthrough" and noted that 69 percent of melanoma patients treated with pembrolizumab were alive after one year.
"The news of FDA's first approval of an anti-PD-1 drug is extremely exciting and shows just how far the field has come in the last few years," said Debra Black, MRA co-founder and chair of the board.
Advertisement
The other recent FDA approvals for melanoma include ipilimumab (2011), peginterferon alfa-2b (2011), vemurafenib (2011), dabrafenib (2013), and trametinib (2013).
Advertisement
Tom Stutz, a patient whose melanoma had spread throughout his body, was among those who enrolled in a clinical trial at the University of California, Los Angeles (UCLA).
"The drug saved my life, that is the bottom line," he said.
Source-AFP