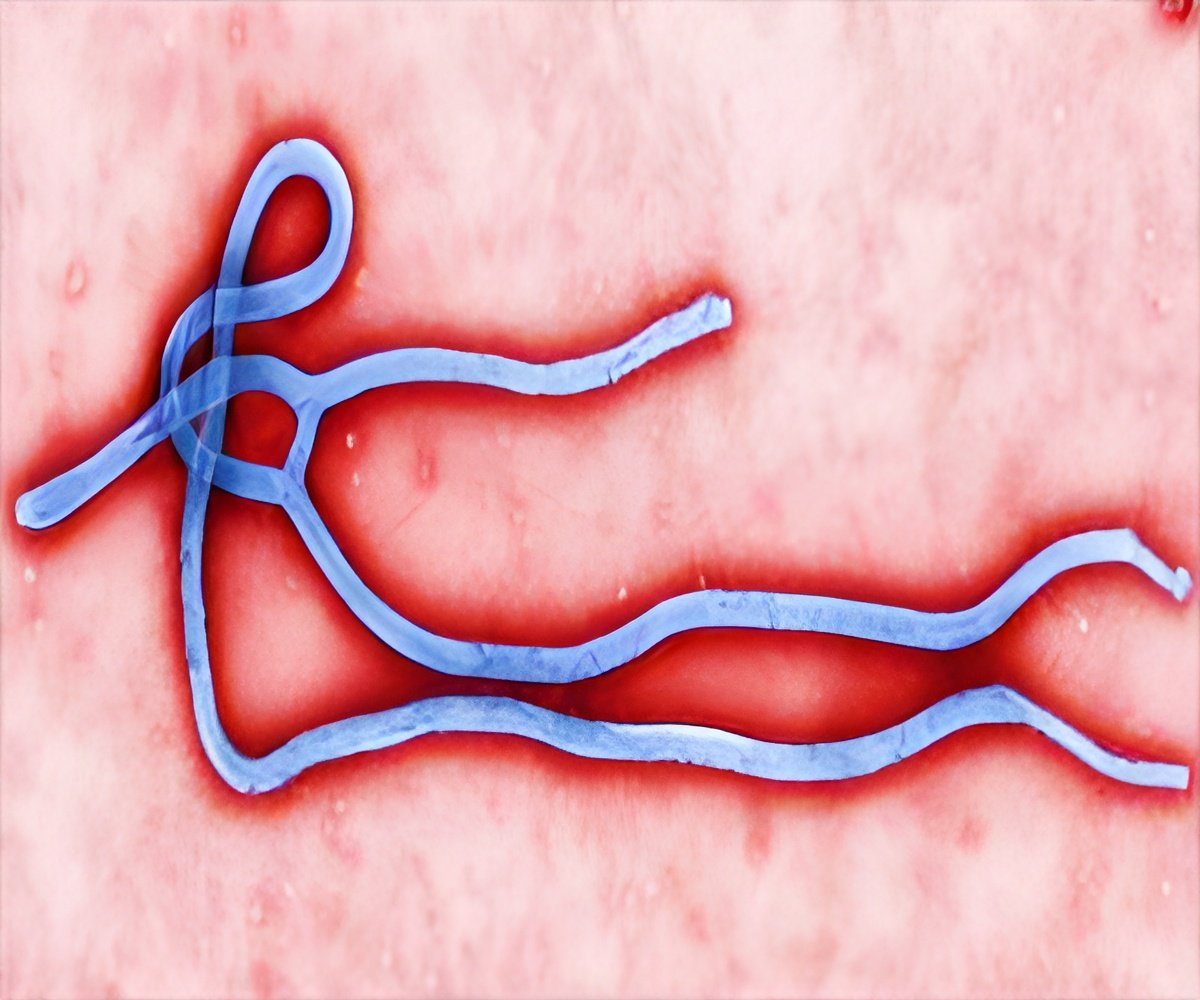
There is no licensed vaccine against Ebola which has killed more than 10,000 people in West Africa out of nearly 25,000 infected cases since the start of 2014, mainly in Liberia, Sierra Leone and Guinea. Researchers are trying to find a safe and effective vaccine against the deadly Ebola virus. Latest reports published in
The Lancet claim that a new vaccine dubbed Ad5-EBOV has proven to be safe in an early trial in healthy adults in China. Researchers said, "But while it triggered an antibody response in test subjects, further trials must be held to establish whether the drug actually protects against Ebola."
The paper said that up to now, all tested Ebola virus vaccines have been based on the virus strain from the Zaire outbreak in 1976. But, this new vaccine is the first based on the strain of the Ebola virus behind the west African outbreak. Study authors from a variety of research institutions, public health bodies and the Tianjin CanSino Biotechnology company, said, "The Phase I trial tested the safety of the drug in 120 people in China's Jiangsu province in December and January."
During the study people were given a low or high dose of the test vaccine, or a placebo. The researchers then monitored the study participants over 28 days for any side-effects and an immune response through the activation of so-called T-cell infection-fighting white blood cells. The researchers said, "We witnessed no serious adverse events and antibodies were significantly increased in participants. This showed the drug has some potential, though it was not known whether the immune response lasted beyond 28 days, or whether a booster shot would be needed."
Source-Medindia