‘Bausch & Lomb's Hypersonic Vitrectomy System is the new device that aids in clearing blood clots in the eye without damaging the retina.’
Tweet it Now
C. Vanitha is now a happy woman. Her left eye was filled with blood, and she had noticed the change in June, after which she could not see at all. But now she has been completely healed at the Dr. Agarwal’s Eye Hospital.“I was nervous when I was told that I was undergoing a new procedure, but now my vision is completely restored,” ” said the 40-year-old.
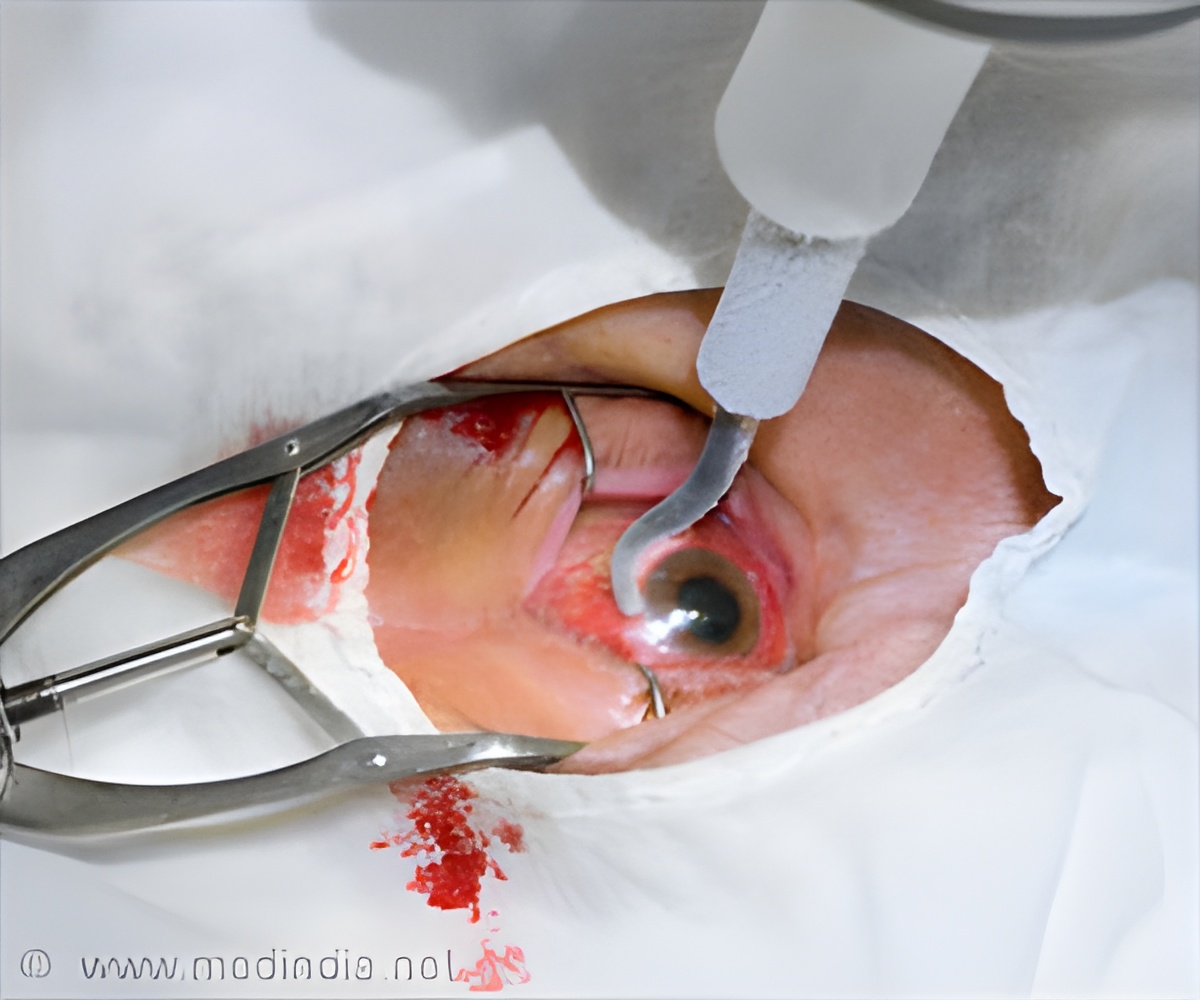
A new device called Bausch & Lomb’s Hypersonic Vitrectomy System, which is a New Stellaris Elite Vision Enhancement System was used to treat Ms. Vanitha, who had hypersonic vitrectomy, said the chairman of the hospital, Amar Agarwal.
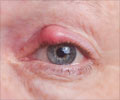
Bleeding in the Eye
Bleeding in the eye occurs due to uncontrolled diabetes, hypertension or injury. Doctors say that removing blood clots from the eye is quite a challenge and the retina could be fixed using a cutter, and this device performs 5,000 cuts per minute creating traction, and pulls on the retina.
Advertisement
The new device reduces the traction, making it the safest procedure. There are also no damages done to neighboring structures in the eye.
Advertisement
Dr. Agarwal’s Eye Hospital, Amar Agarwal, chairman said, “Technology for performing vitrectomy has changed little over the last 40 years, Vitesse would be able to provide a safer option to patients as it would help with less energy usage during retinal surgeries which would minimize the chances of injury this would be the first and only hypersonic device for vitreous removal which is approved by the Food and Drug Administration (FDA) of USA,” he added.
Sanjay Bhutani, managing director, Bausch & Lomb India said, “There have been 22 surgeries done so far, and all of them were successful.”
Source-Medindia