A recent device is ready for trial in the U.S which can be a boon for precision ablation of prostate tumors and this could be a good shot in the arm for clinicians.
The NanoKnife from AngioDynamics specializes in irreversible electroporation which is nothing but initiating a powerful electric field with a combination of electrodes.
This device enables the entry of ions into the cell interiors and leads to permanent necrosis.
AngioDynamics has not received the FDA approval but they have been given the go ahead with an Investigational Device Exemption (IDE) trial of the NanoKnife for focal treatment of prostate cancer.
Source-Medindia
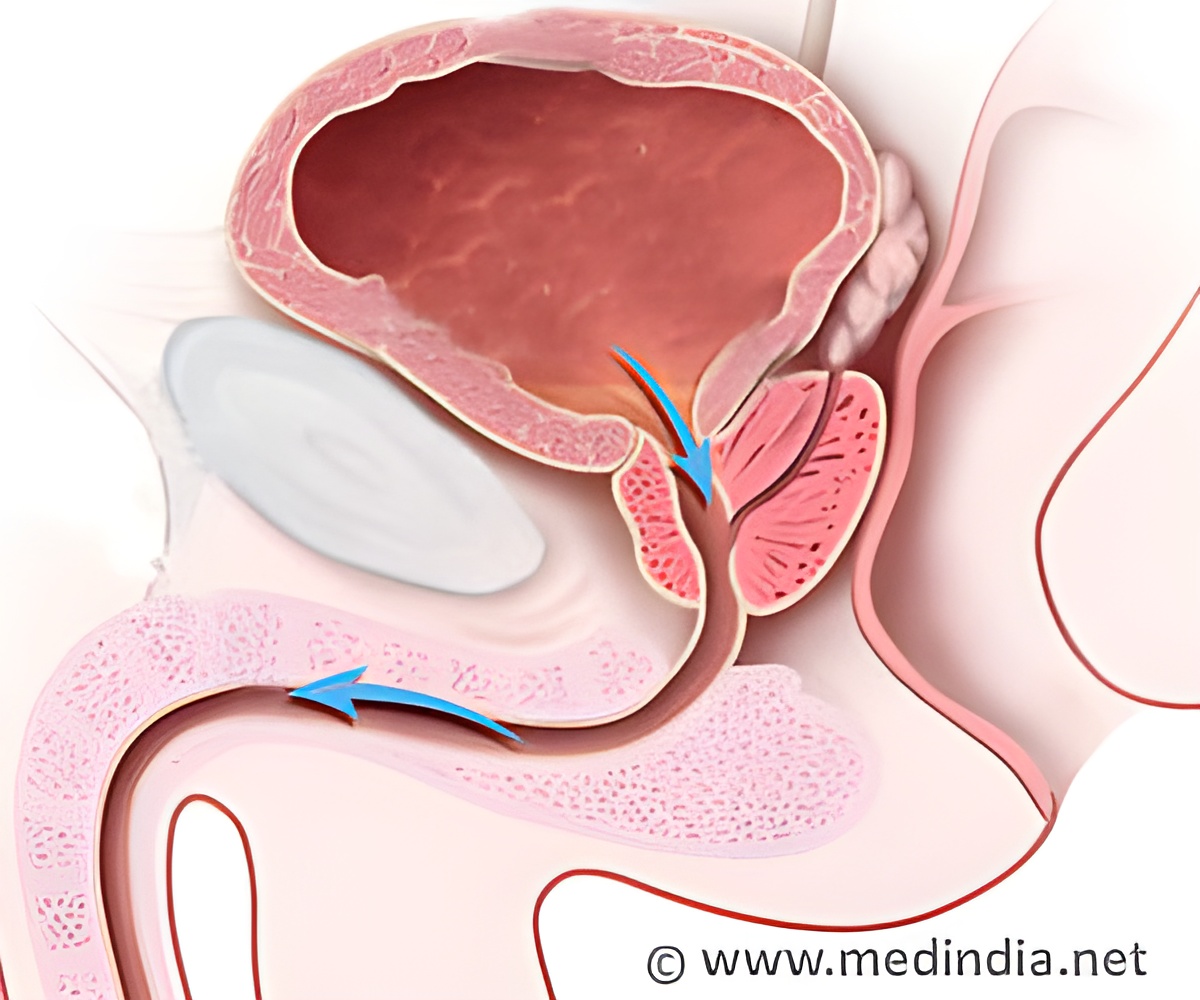

![Prostate Specific Antigen [PSA] & Prostate Cancer Diagnosis Prostate Specific Antigen [PSA] & Prostate Cancer Diagnosis](https://www.medindia.net/images/common/patientinfo/120_100/prostate-specific-antigen.jpg)