Pediatric high-grade glioma is the primary cause of cancer death in children. These pediatric tumors are believed to be caused due to mutations in proteins that disrupt the development of the brain. However, lack of reliable animal models limits our understanding of these tumors. New study has established a novel laboratory model that replicates key hallmarks of this disease. The study was conducted by research teams from the German Center for Neurodegenerative Diseases (DZNE) together with colleagues from Canada and the UK. The study published in the journal
Cancer Cell paves the way for a better understanding of processes, relevant for both cancer and neurodegeneration.
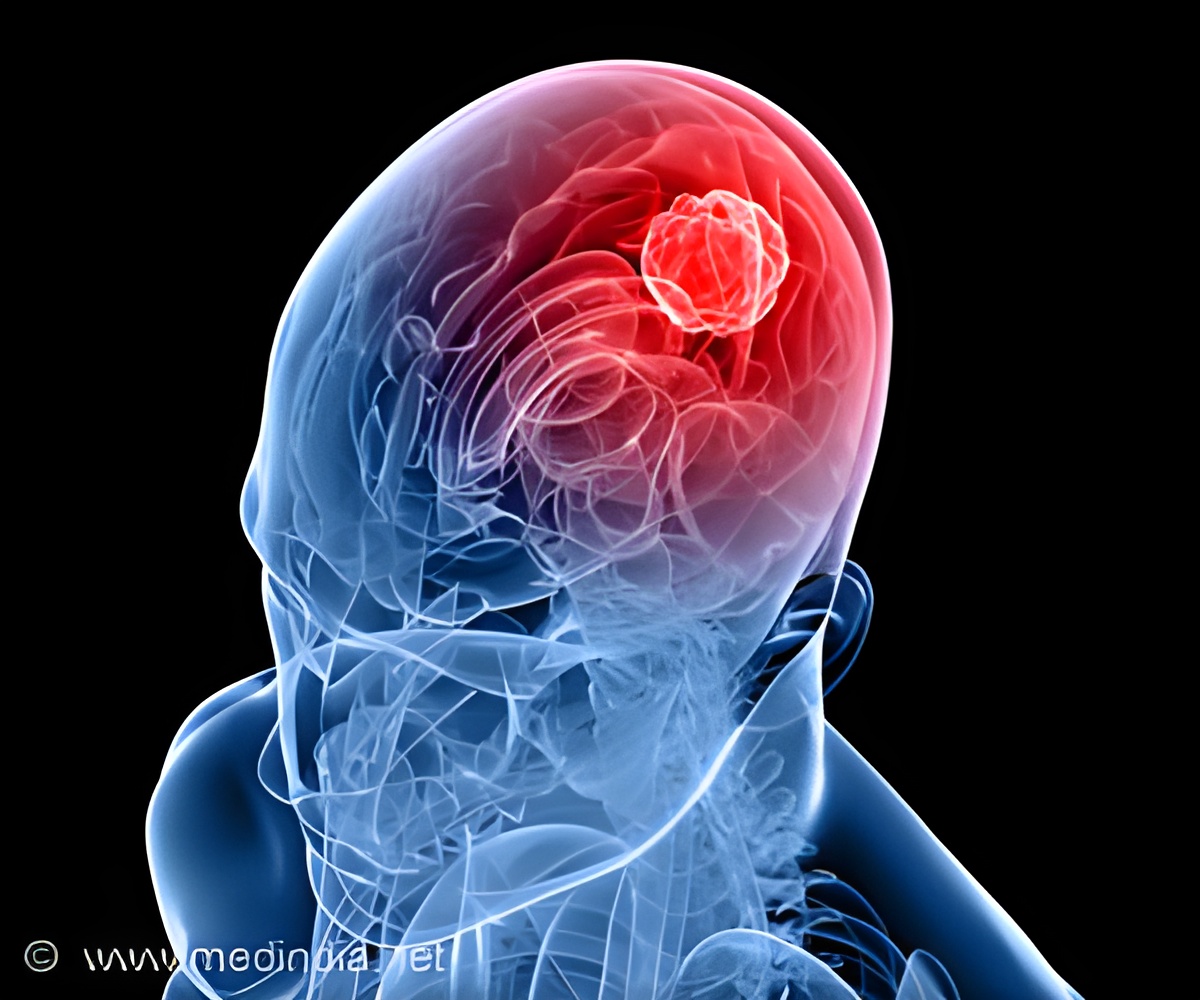
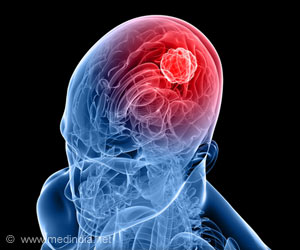
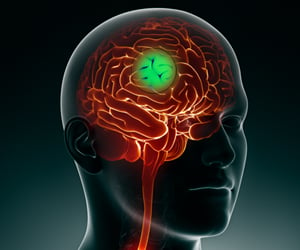
Pediatric high-grade glioma (pHGG) is a devastating illness and the most deadly cancer affecting children.
Mutations in "histone 3.3", a DNA-binding protein that acts upon gene expression for regulation of brain function and aging, are considered to play a pivotal role for the development of these tumors. "Current treatment involves surgery, radiation and chemotherapy, albeit with limited success. Most patients die within one to two years from diagnosis," says Prof. Paolo Salomoni, who leads a research group at the DZNE's Bonn site. For the current study, his team cooperated with the lab of Prof. Nada Jabado at McGill University in Montreal and with researchers from other institutions.
‘Mutations in histone 3.3 alter gene regulation during embryonic development, which indicates that the cancer likely starts in utero.’
"
Up to now, there was no truly representative in vivo model to study the underlying mechanisms of this disease," says Salomoni. "That is why we decided to develop a mouse model that recapitulates hallmark pathological features of pHGG. Our findings support the concept that mutations in histone 3.3 alter gene regulation already during embryonic development. This means that the cancer likely starts in utero."
For the study the researchers altered the blueprint of histone 3.3 in mice by genetic engineering. "This model will enable insights into the development of pHGG and provide an opportunity to explore novel therapeutic approaches", Salomoni says. The biologist sees further potential for applications: "Laboratory experiments from DZNE and others suggest that alterations in histone 3.3 are implicated not just in brain tumors but also in depression and age-related brain diseases. Our model might therefore help to study DNA associated mechanisms involved in a wide spectrum of diseases."
Source-Eurekalert