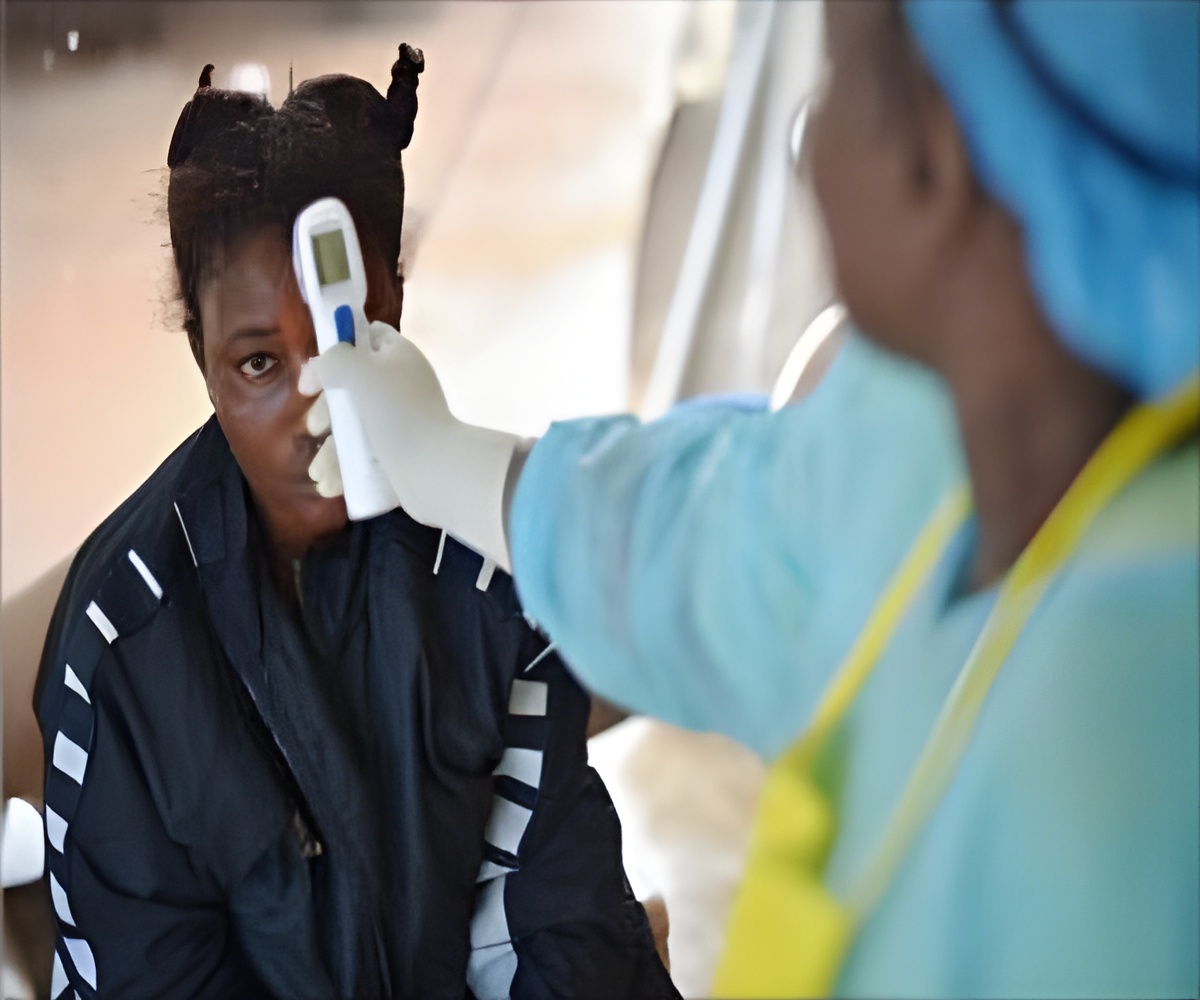
The Ebola test, manufactured by German company TIB MOLBIOL GmbH, has also been given the European CE mark indicating it abides with the EU regulations.
Chief operations officer of Roche's diagnostics division, Roland Diggelmann said, "The LightMix Ebola Zaire test is an easy-to-use molecular diagnostic test providing a solution for healthcare professionals to quickly detect the virus and start patient treatment as early as possible.”
Source-Medindia