‘Cilostazol is a unique antiplatelet agent. As a phosphodiesterase III inhibitor, it improves endothelial function, restricts the clumping of blood cells (platelet aggravation), widens blood vessels (vasodilator), and mildly inhibits cell growth.’
Tweet it Now
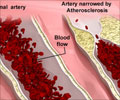
The blockage of a neck artery (carotid artery) is a major cause of stroke. Opening the carotid artery with a mesh tube known as a stent is an effective treatment. However, patients can develop a blockage - known as in-stent restenosis, which may increase the risk of recurrent stroke.Cilostazol is FDA-approved to treat leg pain in people with peripheral vascular disease.
"This is the first trial to show potential effectiveness of medical management for the prevention of in-stent restenosis after carotid artery stenting," said Hiroshi Yamagami, M.D., Ph.D., lead study author and director of the Department of Stroke Neurology at National Hospital Organization Osaka National Hospital, Japan.
The Carotid Artery Stenting with Cilostazol Addition for Restenosis (CAS-CARE) study is a multi-center, prospective, randomized, open-label trial evaluating the inhibitory effect of cilostazol on in-stent restenosis, compared to other antiplatelet medications in patients scheduled to undergo carotid artery stenting.
Eligible patients were randomly assigned to receive cilostazol (50 mg or 100 mg, twice per day), or any antiplatelet agents other than cilostazol, starting three days before stenting and continued for two years. A total of 631 patients (average age 70, 88% of men) were included in the full study analysis. In-stent restenosis occurred in 9.5% of patients in the cilostazol group and 15% of patients in the non-cilostazol group during two years of follow-up.
Advertisement
Source-Eurekalert