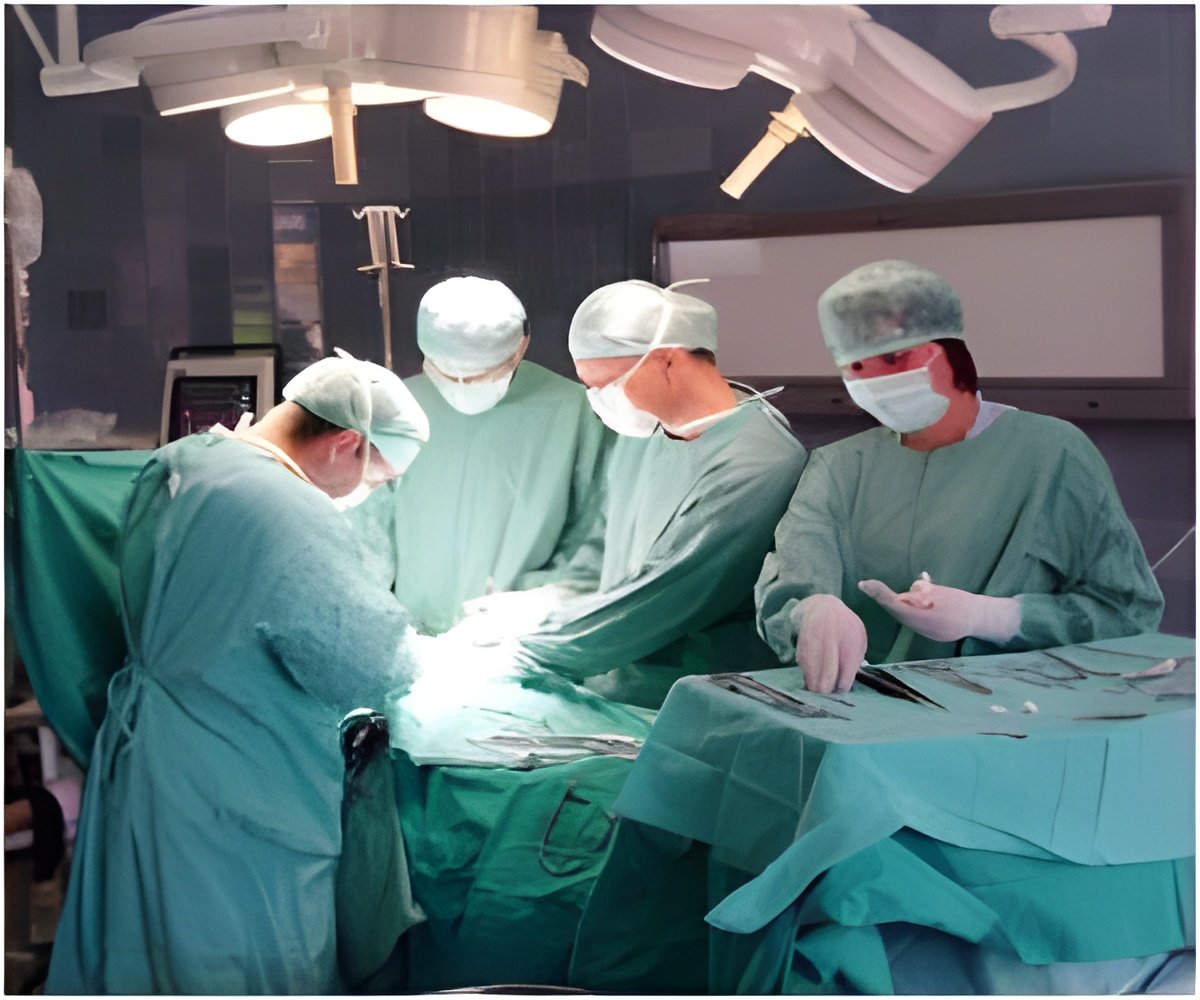
New Jersey-based medical devices maker Integra LifeSciences revealed that its Integra Flowable Wound Matrix device has received European CE Mark Certification.
The device has been approved for filling deep soft tissue or tunneling wounds, including diabetic foot and leg ulcers in the European Union. The company also said that the device has won the innovation contest held by the French Society of Reconstructive and Esthetic Plastic Surgery.
Stating that the device offers new options for both the doctors and patients, Integra’s Debbie Leonetti said, “Tens of thousands of patients in Europe suffer from chronic foot and leg ulcers that progress to deep or tunneling wounds, and they face serious risk of amputation. Integra's Flowable Wound Matrix now offers surgeons, and patients, a new option for managing these hard-to-access wounds.”
Source-Medindia