One of them said that the drug needs to be closely examined for adverse events and if the number goes high then it should be notified to the regulatory agencies. "It is particularly concerning that the product is showing these reactions very shortly after its commercial launch. We want indigenously made products, but patient safety is paramount," he said.
Intas sought to downplay the issue and attributed the reported reactions to inadequate maintenance of cold chain in its new distribution vertical. The company claimed that it has a robust pharmacovigilance program and it adheres to the norms related to pharmaceutical safety update reports or PSURs.
To ensure complete logistic uniformity and absolute maintenance of the cold-chain, Intas said it has curtailed the drug's distribution, supplying it to restricted centers. "We are further strengthening (our) cold chain by maintaining data loggers for temperature with each individual delivery of Razumab. This would continuously record the temperature and ensure instant validation of cold chain to offer complete solution," the company said.
According to industry sources, the Vitreo Retina Society-India (VRSI), a body of super specialty doctors in the field of ophthalmology, recently issued a cautionary letter to its members about the adverse reactions seen in Razumab.
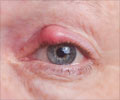
The two experts quoted earlier said that about nine cases of endophthalmitis, or inflammation of the inner coats of the eye, was reported.
Advertisement
The firm, however, said that as an additional check it recalled a few units of commercial stock randomly and analyzed them at its quality control department. "We have found that all Razumab stock retested is stable and as per the QC requirement. The company will supply the product widely only where we have assured adherence to cold chain", it said.
Advertisement