Rosiglitazone is an anti-diabetic drug belonging to the thiazolidinedione group of drugs. Its major role is in insulin sensitization and making insulin more easily available to the cells. It does this by binding the PPAR (Peroxisome-Proliferator–Activated 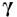 Receptors) to adipose tissue and this action makes the cells more receptive to the insulin hormone. Insulin is secreted by pancreas and is responsible in the body for reducing blood sugar. In diabetes the insulin is either in short supply or the tissue cells or insensitive to the circulating insulin.
Receptors) to adipose tissue and this action makes the cells more receptive to the insulin hormone. Insulin is secreted by pancreas and is responsible in the body for reducing blood sugar. In diabetes the insulin is either in short supply or the tissue cells or insensitive to the circulating insulin.
It has been observed that the prevalence of obesity, imbalanced glucose tolerance, and type 2 diabetes mellitus is ascending at an alarming rate. If the current trends continue, approximately 33% of adults in the United States may develop diabetes by the year 2050. The situation is likely to be a lot worse in countries like China and India. All this is a highly alarming prediction which calls for need for some new, more effective, and safer strategies for the prevention and treatment of diabetes.
Recently researchers reported findings of some studies that may empower the development of safer anti-diabetic agents which would target peroxisome-proliferator–activated receptor 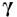 (PPAR
(PPAR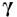 )
). According to the study, therapeutic effect of rosiglitazone is facilitated by its prevention of the phosphorylation of PPAR
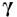
.
Synthetic activators of PPAR
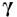
(thiazolidinediones) act primarily as effective insulin sensitizers and helps in reducing plasma insulin levels as well as the complications associated with hyperinsulinemia. The prominent side effects of thiazolidinediones group of drugs to which the rosiglitazone belongs included weight gain, congestive heart failure, fluid retention, and bone fractures. The use of these drugs became controversial when the side effects surfaced more evidently. Earlier in this year, the FDA banned the usage of the most commonly prescribed thiazolidinediones (rosiglitazone) because of a steep rise in the rates of complications from cardiovascular causes.
The metabolic effects of thiazolidinediones are because of the transcriptional regulation in fat cells and other tissues which affect whole-body insulin sensitivity. Although the actual mechanisms of the insulin-sensitizing effects of these drugs are unknown but still the classic agonist effects of thiazolidinediones include the induction of an adipogenic gene program. Adipocytes play important role in metabolic regulation by storing the lipids and as a result reduce the ectopic lipid accumulation in other tissues. A better understanding of the effects of thiazolidinediones on adipocytes could provide an insight for developing much safer agents which targets PPAR
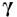
, preserves the insulin-sensitizing effects, and diminish the detrimental side effects as well.
Dr. Jang Hyun Choi of Harvard Medical School, who was a researcher on the study, found that the serine residue at position 273 of PPAR
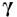
is phosphorylated by cyclin-dependent kinase 5 (CDK5) and its phosphorylation at this position alters its transcriptional activity. This results in the reduced expression of many genes that promote positive metabolic effects, most prominently insulin sensitivity. He found that CDK5 is activated by proinflammatory cytokines that are elevated in fat cells of obese people and in response to consuming a diet high in fat. This suggests that CDK5-altered PPAR
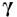
transcription to some degree attenuates the beneficial effects of adipose tissue on whole-body metabolism.
Rosiglitazone activates PPAR
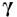
-controlled transcription and inhibits CDK5 phosphorylation of PPAR
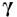
. This inhibition restores the expression of adipocyte genes, which are important for the beneficial metabolic effects of fat cells. The antidiabetic activities of many non-thiazolidinedione PPAR
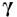
ligands correlate much better with the inhibition of CDK5 phosphorylation of PPAR
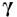
than with the induction of adipogenesis. In a small group of people who has impaired glucose tolerance and were treated with rosiglitazone, the decrease in the phosphorylation of PPAR
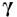
on serine residue 273 correlated with the degree of improved insulin sensitivity. It will be important to seek if the inhibition of CDK5-induced phosphorylation is a major cause (or result) of the therapeutic effect of rosiglitazone treatment and whether such inhibition is mandatory for the insulin-sensitizing effects of rosiglitazone.
The data from the study revealed that modifying the transcriptional machinery to fine-tune the expression of specific gene sets may enhance therapeutic efficacy and helps in diminishing the side effects of drugs that target nuclear receptors. Similar goals have led to the development of selective agonists of the androgen receptor, estrogen receptor, and thyroid hormone receptor, although the mechanisms conferring specificity of these agonists differ for the various receptors. Array studies show overlapping but distinct profiles of gene expression associated with the use of different thiazolidinediones, and clinical studies show varying effects of individual thiazolidinediones on plasma lipoproteins, henceforth reinforcing the feasibility of developing selective PPAR
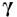
agonists.
The study by Dr. Choi and fellow researchers suggests a particular approach for developing selective PPAR
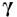
ligands, targeting CDK5 phosphorylation of PPAR
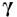
or the selective downstream effects of this phosphorylation.
Although it is too premature to conclude that CDK5 phosphorylation of PPAR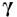 has a critical role in normal physiology/pathophysiology of obesity and insulin resistance, these findings do provide proof of concept that the transcriptional effects of PPAR
has a critical role in normal physiology/pathophysiology of obesity and insulin resistance, these findings do provide proof of concept that the transcriptional effects of PPAR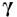 ligands can be separated from the effects underlying insulin sensitization
ligands can be separated from the effects underlying insulin sensitization. Still, it will be imperative to determine whether the side effects and therapeutic effects can be separated from each other.
Now the big question arises if it be wise to develop more PPAR
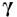
ligands when other types of anti-diabetic drugs are in the pipeline?
The answer to this is Yes. The reason being even in the absence of excessive diabetes,
insulin resistance offers an increased risk of cardiovascular disease and cancer. The vast majority of obese people have insulin resistance and hence are at risk for such complications. Safer insulin sensitizers could be used to prevent the development of diabetes and cardiovascular disease in persons with insulin resistance.
Source-Medindia
![]() Receptors) to adipose tissue and this action makes the cells more receptive to the insulin hormone. Insulin is secreted by pancreas and is responsible in the body for reducing blood sugar. In diabetes the insulin is either in short supply or the tissue cells or insensitive to the circulating insulin.
Receptors) to adipose tissue and this action makes the cells more receptive to the insulin hormone. Insulin is secreted by pancreas and is responsible in the body for reducing blood sugar. In diabetes the insulin is either in short supply or the tissue cells or insensitive to the circulating insulin.