Highlights
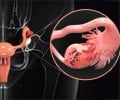
- Ovarian cancer is the sixth most common cancer in women.
- A combination of drugs that include PARP inhibitor (Poly ADP ribose polymerase inhibitor) along with a kinase inhibitor has been found to treat ovarian cancer.
- Olaparib (PARP inhibitor) along with a P13-kinase inhibitor drug is found to show a good duration response for 5.5 months.
Research Study
The research study was conducted with twenty-eight patients who had high-grade serous ovarian cancer. Olaparib drug is a PARP inhibitor which is given along with an investigational alpha-specific P13-Kinase inhibitor, BYL719 in phase I trial.
Out of the 26 patients, 28 of them had platinum-resistant cancer. Konstantinopoulos, medical oncologist said, that such patients responded to a PARP inhibitor for as low as 4%
Pre-clinical studies showed that adding a P13K inhibitor may sensitize the cancer cells to the effects of the PARP inhibitor and may impair the ability of tumor cells to repair the DNA damage.
Study Findings
The findings of the study showed that the median duration of the response in the ovarian cancer patients was about 5.5 months.
Four of the 28 patients discontinued the therapy due to toxicity.
The drug is approved for treating platinum-resistant ovarian cancer in women with germline breast cancer gene mutation. The drug mainly acts by killing the cancer cells.
Side effects
- Nausea
- Vomiting
- Diarrhea
- Decreased appetite
- Breathing difficulties
- Sleep disturbances
- Weakness
- Pale skin
Konstantinopoulos said, "The activity of this combination in ovarian cancer patients without germline BRCA mutations and with platinum-resistant disease was higher than expected from olaparib monotherapy and warrants further investigation."
References
- Olaparib - ( https://medlineplus.gov/druginfo/meds/a614060.html)
Source-Medindia