- Spinal muscular atrophy (SMA) a genetic disorder that affects the control of muscle movements.
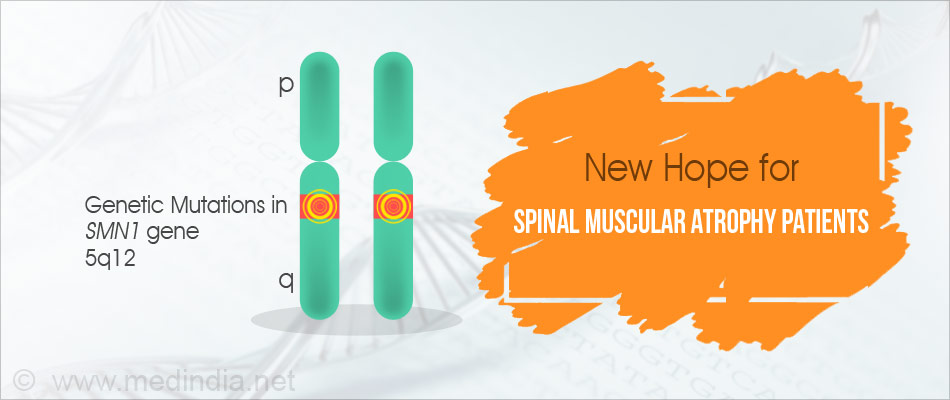
- Genetic mutations on chromosome 5 in SMN1 gene cause the deficiency of survival of motor neuron (SMN) protein which is the characteristic of spinal muscular atrophy (SMA).
- In infantile-onset SMA, activities like sucking, swallowing, sitting, head control, rolling over and other key developmental milestones are affected.
- Nusinersen, an investigational treatment works by increasing the production of SMN protein has shown to significantly improve the motor milestones in affected infants.
The protein is necessary for normal functioning and maintenance of motor neurons. The degree to which the motor neurons are affected, determines the age of onset of symptoms. For children who display symptoms at birth or in the first few months of life, there is a greater impact on the motor neurons and have the lowest level of functioning ( Type 1- the infantile onset SMA known as Werdnig-Hoffman disease).
Infants affected with SMA have a weak cry and distressed breathing. They have difficulty in sucking or swallowing and do not hit the developmental milestones like rolling over or sitting up independently. They do not survive past two years of age.
Interim analysis of double-blind, randomized, placebo controlled Phase 3 clinical trial called ENDEAR, has shown that use of nusinersen, an investigational treatment for SMA, has helped to significantly improve the achievement of developmental milestones in affected infants.
Nusinersen is designed to increase the production of fully functional SMN protein by regulating gene expression. It is incorporated into the fluid around the spinal cord through lumbar puncture or spinal tap. Multiple doses may be required.
The sponsoring company, Biogen, is working to globally expand the program and make it accessible to all patients with infantile-onset SMA. 36 centers around the world have participated in the study. Ann & Robert H. Lurie Children's Hospital of Chicago had the highest patient enrollment.
“Going forward, we could add SMA to the newborn screening panel and treat infants in the pre-symptomatic phase,” said Leon Epstein, MD, Co-PI at Lurie Children's, Division Head of Neurology, Medical Director of the Clinical Research Unit of Stanley Manne Children's Research Institute at Lurie Children's, and Professor of Pediatrics and Neurology at Northwestern University Feinberg School of Medicine.
The success in the clinical trial is a source of hope for the families of affected infants as well as the physicians treating them.
References:
- What is Spinal Muscular Atrophy? - (https://www.mda.org/disease/spinal-muscular-atrophy)
- Genetics of Spinal Muscular Atrophy - (https://ghr.nlm.nih.gov/condition/spinal-muscular-atrophy)