"Effective treatment of RA is essential to control disease progression and improve quality of life for patients," says Dr. Jasvinder Singh with the University of Alabama at Birmingham, the principal investigator for the 2012 update of the ACR RA guidelines for the use of DMARDs and biological agents. "With additional advancements in RA therapies since 2008, it was important to update recommendations that help guide rheumatologists in treating RA patients receiving DMARDs or biologic therapies."
The 2012 DMARD and biologic agents recommendations included a number of areas but concentrated on four updated sections:
- Indications for use and switching of DMARDs and biologics
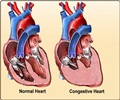
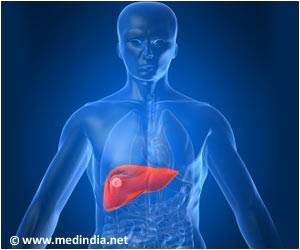
- Use of biologic agents in high-risk RA patients with hepatitis, cancer, or congestive heart failure
- Screening for TB in RA patients starting or receiving biologic drugs
- Vaccination in patients starting or receiving DMARDS or biologics
Dr. Singh further explains, "The recommendations for DMARD and biologic treatment provide a guide for rheumatologists who care for RA patients. However, these guidelines should not replace important physician-patient discussions or individual clinical decisions that take into account assessments of risk-benefits, patient preferences, and economic considerations."
The authors suggest that low disease activity or remission should be the goal for each RA patient, but each patient's therapy target should be specific to their particular health needs. One of the noted changes from the 2008 guidelines is more aggressive treatment in patients with early RA that is within six months of symptom onset. Researchers believe the recommended change to more intensive early therapy is that earlier treatment may provide better outcomes; joint damage in RA is irreversible, making prevention of damage an important goal; and preserving physical function and health-related quality of life is necessary to reduce disability.
A related editorial is also available today in Arthritis Care & Research. Co-author Dr. David Daikh, Associate Professor of Clinical Medicine at the University of California, San Francisco and Chief of Rheumatology at the VA Medical Center comments, "The treatment of RA is a rapidly changing field with new therapies regularly becoming available. As this field evolves, the recommendations for treatment with DMARDs and biologic drugs will need to be modified in the future and the ACR must be nimble in keeping these guidelines as current as possible."
Advertisement