‘Brain swelling in stroke patients can be reduced by glyburide injections.’
Tweet it Now
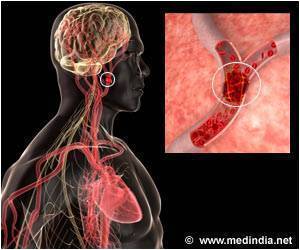
Accumulation of excess fluid in the brain may cause brain swelling. This usually occurs during stroke which is mainly due to a blockage in the blood supply to the brain. Swelling in the brain may increase pressure and pushes the brain out of the skull resulting in 50% of the mortality rate. Surgery procedures like hemicraniectomy ( removing a portion of the skull to reduce swelling) may not be possible with all patients and treatment using drugs may also be less effective.According to previous animal studies, glyburide used in the treatment of diabetes was found to reduce brain swelling in stroke patients. The phase 2 clinical trial for glyburide was sponsored by Remedy Pharmaceuticals following a pilot study conducted by Kimberly and co-author Kevin Sheth, MD, Department of Neurology, Yale University School of Medicine, which reported that glyburide was found to be safe for stroke patients.
Glyburide Advantage in Malignant Edema and Stroke (GAMES-RP) trial was conducted in 18 hospitals across United States. About 77 stroke patients were randomly assigned for continuous glyburide treatment or placebo for 72 hours.
The results were obtained from patients after 90 days, using a standardized stroke scale ranging from 0 to 4 indicating no symptoms to moderate and severe brain swelling. About 40% of the patients met the criteria and were able to survive without surgery.
Sheth said that the decision for performing surgery seems to be complicated and were noted only in cases where physicians decide based on the wishes of the patient or their family. And this was found to be the main reason for not reaching the endpoint of the clinical trial.
Advertisement
Advertisement