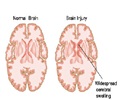
All patients in phase 2 underwent surgery prior to the vaccine therapy, which generally begins within 5 weeks after surgery, and consists of four weekly injections, followed by bi-weekly injections for up to 52 weeks.
The results found these patients to have a median survival of 11 months compared to the current three to five month survival.
"The findings are very favorable for patients with this deadly form of brain cancer. The vaccine is one of the few immune therapies designed specifically for patients who are not newly diagnosed, and these encouraging results make this a promising therapy for a more extensive Phase 3 trial," said Andrew Sloan, MD, one of the authors of the study presented at ASCO and Director of the Brain Tumor and Neuro-Oncology Center at University Hospitals Case Medical Center.
The study was presented at The American Society of Clinical Oncology (ASCO) annual meeting.
Source-ANI