A team led by St. Jude researchers identified a gene pivotal for immune system balance. Ultimately, the discovery may aid efforts to tame allergies and asthma.
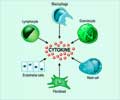
Named Mina, the gene is part of a signaling pathway that may provide novel targets for new treatments and provide further insights into the disease-fighting immune system, explained Mark Bix, Ph.D., Immunology. Bix is senior author of the paper published in Nature Immunology.A healthy immune system requires balance. Bix is focused on the balance of two specialized cells in one branch of the immune system. The cells are called T-helper type 1 (Th1) and T-helper type 2 (Th2). They arise from a pool of immune cells known as naïve T-helper cells that are arrested at a preliminary stage of differentiation where they are poised for a final differentiation step. During that step they are supposed to acquire specialized immune functions optimized for the control of eliciting pathogens. Chemical messengers known as cytokines control the development of naïve T-helper cells into a variety of more specialized cells, including the Th1 or Th2 cells.
That’s where Mina comes in. Researchers reported this gene works indirectly by regulating production of interleukin 4 (IL-4), a cytokine that plays a central role in balancing Th1 and Th2 cells. Using classical genetics and molecular techniques, investigators showed that Mina dampened IL-4 production by binding to a region of DNA known as the IL-4 promoter. The promoter is where IL-4 production begins. Mice that made large amounts of the Mina protein had low IL-4 levels. When Mina was eliminated, production of the cytokine jumped.
Researchers used a variety of tests involving mouse strains that made different levels of IL-4 to show the rise and fall of IL-4 indirectly controlled Th2 production. High levels of IL-4 favor production of Th2 rather than Th1.
The findings come more than a decade after Bix, then a postdoctoral fellow, started looking for why certain mouse strains mounted an immune response that favored production of either Th1 or Th2 cells. Certain diseases are characterized by an imbalance between those cells.
An imbalance favoring Th2 cells, known as Th2 bias, is linked to an increased risk of problems like allergies and asthma. Th2 cells cause the inflammation that is a hallmark of asthma. Th2 bias is also associated with increased susceptibility to the parasitic infection leishmaniasis, which remains a threat in regions around the equator.
Advertisement
IL-4 relies on a positive feedback mechanism to influence Th2 production. Newly activated T-helper cells make a small amount of IL-4. The IL-4 then returns through a pathway that includes the IL-4 receptor and transcription factors STAT6 and GATA-3 to entice more T-helper cells to become Th2 rather than Th1 cells. The new Th2 cells respond by secreting even more IL-4.
Advertisement
In this study, Bix and his colleagues also proposed a reason for the wide variation in Th2 bias between different mouse strains. The researchers pointed to a region of DNA that included the Mina promoter. They decoded the same region in five different mouse strains. Two favored Th2 production and three did not. The scientists eventually linked the differences to 21 single nucleotide polymorphisms (SNPs). SNPs are relatively common variations in the makeup of particular genes. Those SNPs distinguished mice with high numbers of Th2 cells from those with less Th2 bias. The researchers noted the same DNA region might contain other genomic variations that explain differences in Mina activity.
Mina belongs to a particular family of genes known as transcription factors. Cells use transcription factors to help carry out the instructions genes carry. Mina first surfaced in 2002 in connection with human cancer. Those earlier studies suggested Mina was a target of the Myc oncogene, or cancer gene. However, in T helper cells, Bix’s group found no evidence linking Mina expression with Myc activity.
Other questions remain. Mina lacks an obvious location for binding to the IL-4 promoter. Researchers believe another transcription factor, named NFAT, plays a role in the hookup. They noted the Dice1.2 region of chromosome 16 where Mina was found might contain other genes that contribute to Th2 balance. Mina’s role in response to a Leishmania major infection also remains unclear. Earlier research linked response to the infection to the same DNA region where Mina was found. Researchers speculated Mina might also be the foundation of that response.
Source-Newswise
SRM