Patients above 18 years are advised to wear the device for no more than 20 minutes per day. The device, already sold in Canada, costs $250. “Cefaly provides an alternative to medication for migraine prevention,” said Christy Foreman, director of the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health.
The device is put in the centre of the forehead, just above the eyes. The device sends an electric current to the skin and underlying body tissues to stimulate trigeminal nerve, which is linked to migraine headaches. Thus, the device aids in producing more endorphins in the brain which helps in checking migraine.
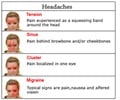
Migraine patients suffer from throbbing pain in one area of the head. It also causes nausea or vomiting and sensitivity to light and sound. Migraine headaches can affect a person from four to 72 hours. As per National Institutes of Health, approximately 10 per cent people across the globe suffer from migraine.
The device got approval after evaluating the data of 67 individuals in Belgium who got more than two migraine headache attacks a month.
Source-Medindia