FDA announced revisions to the labels of certain epilepsy, bipolar disorder and nerve pain medications to strengthen warnings about potential risk for rare skin disorders and recommended that patients with Asian backgrounds undergo genetic tests before they take the treatments, the Wall Street Journal reports.
The revisions apply to:- Carbamazepine, the active ingredient in Carbatrol, manufactured by Shire;
- Tegretol, manufactured by Novartis; and
- Equetro, manufactured by Validus Pharmaceuticals.
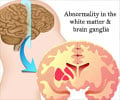
According to FDA, about one to six per 1,000 patients who begin to take the medications will develop the skin disorders in nations with largely white populations, with a 10% increase in risk in nations with largely Asian populations. FDA said that studies have found a link to the skin disorders and an inherited variant of HLA-B 1502, a gene found almost exclusively in individuals with Asian backgrounds.
In addition, FDA said that patients who have taken the medications for more than a few months and have not developed the skin disorders likely will not develop them, regardless of background.
"Patients currently taking carbamazepine who are concerned about these skin reactions should not stop taking the drug without first consulting their health care provider," FDA said.
Source-Kaiser Family Foundation
SRM/P