The panel urged the drug's approval despite mixed results on its effects at different doses, and some safety concerns about the risks of cancer -- mainly lymphoma -- and infections.
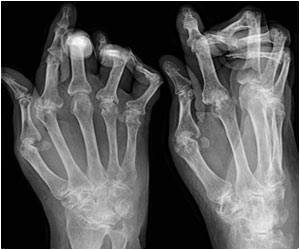
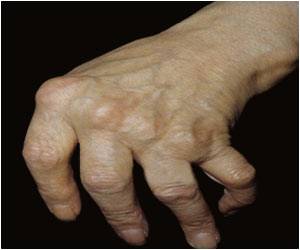
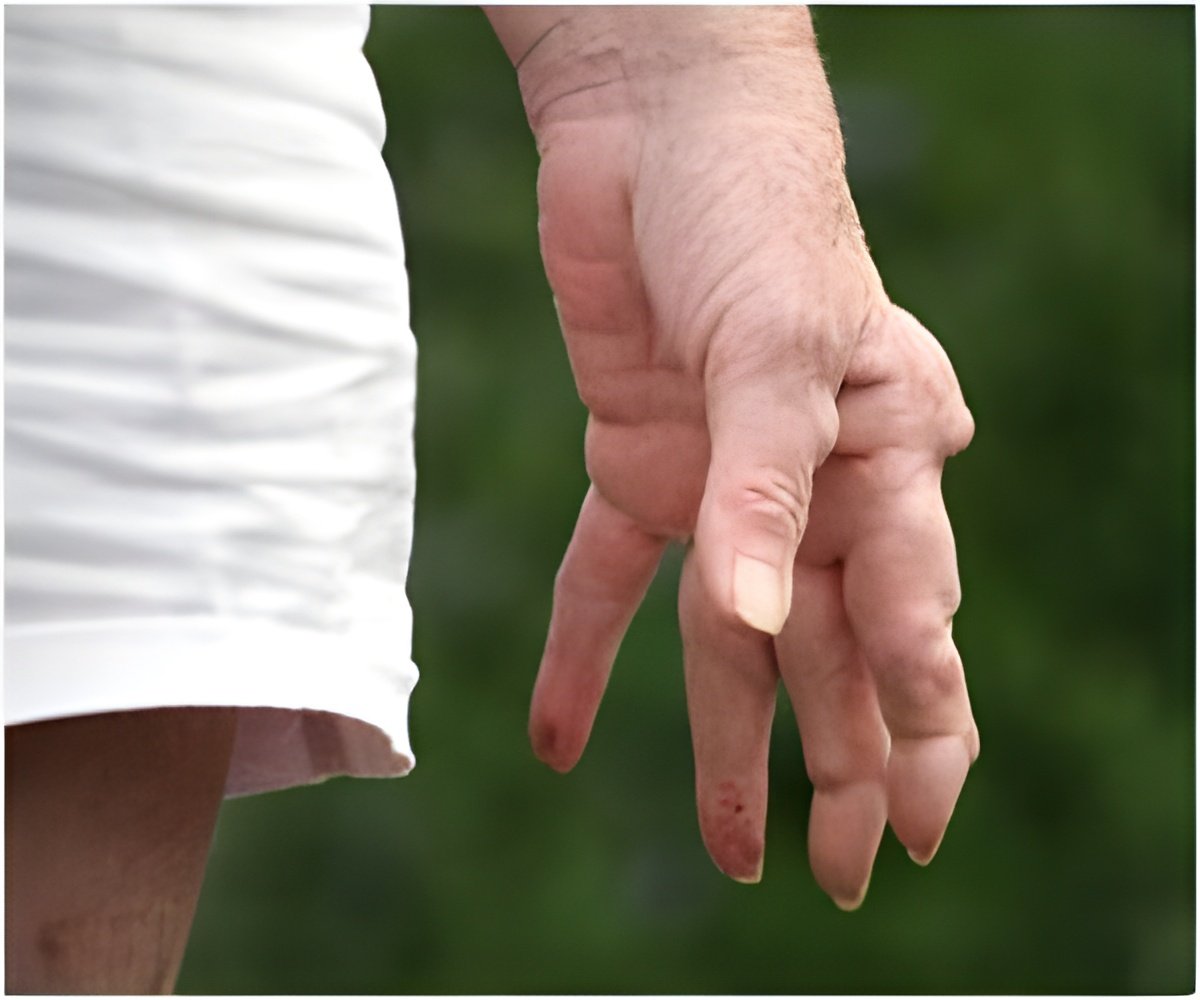
Rheumatoid arthritis is a progressive autoimmune disease that causes debilitating joint pain that can lead to deformity, and affects about one percent of people in the United States and Europe, most of them women.
Exactly what causes the disease remains a mystery, and there is no known cure.
The FDA does not have to follow the recommendations of the advisory panel though it often does.
Source-AFP