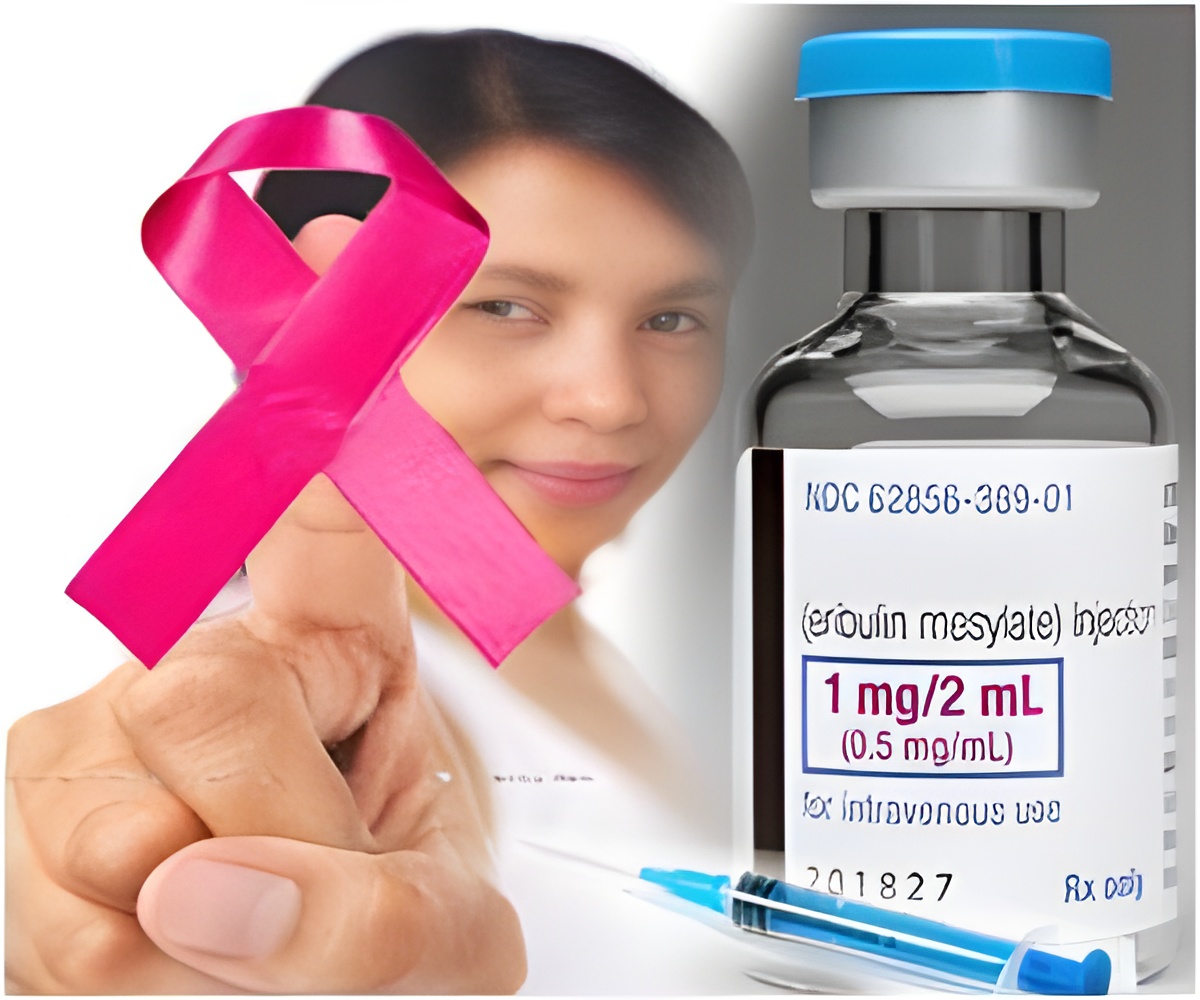
The US Food and Drug Administration has approved a new therapy developed by Roche which it hopes will prove beneficial for patients suffering from HER2-positive, late-stage breast cancer.
The therapy, known as Kadcyla, has been approved for patients who have already been treated with another anti-HER2 therapy, trastuzumab, along with chemotherapy drug, taxanes. “Kadcyla delivers the drug to the cancer site to shrink the tumor, slow disease progression and prolong survival”, FDA’s Dr Richard Pazdur said.
The FDA revealed that clinical trials conducted on a group of 991 patients should that Kadcyla increased the progression free survival period to 9.6 months compared to 6.4 months in patients treated with lapatinib plus capecitabine while the overall survival period was 30.9 months compared to 25.1 months.
Source-Medindia