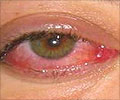
Presbyopia is a vision condition in which the crystalline lens of your eye loses its flexibility, which makes it difficult for you to focus on close objects. It occurs with normal aging and results in difficulty with near vision, generally in adults 40 to 50 years of age.
William Maisel, M.D., deputy center director for science in the FDA’s Center for Devices and Radiological Health said that the device provided a new option for correcting near vision in certain patients.
“The device works by blocking unfocused light rays entering the eye in order to improve near vision. It blocks peripheral light rays while allowing central light rays to pass through a small opening in the center of the device, making near objects and small print less blurry,” Maisel said.
In the surgical procedure, a laser is used to create a pocket in the cornea of one eye of the patient and implants the device in that pocket. This is intended to allow the patient to have improved near vision in the eye containing the implant, while not affecting the distance vision of the two eyes working together.
The FDA reviewed the results of three clinical studies to evaluate the safety and efficacy of the device. The results of the main study showed that participants achieved a level of vision that is needed to read most text in magazines and newspapers.
Advertisement
Source-Medindia