Highlights
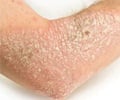
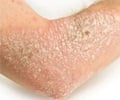
- Psoriasis is a skin condition which causes redness and scaly patches on the skin
- The Food and Drug Administration (FDA) approved brodalumab drug for psoriasis treatment
- Brodalumab drug found to be effective in the treatment of moderate-to-severe plaque psoriasis. The drug is available only through Risk Evaluation Mitigation Program (REMS)
Julie Beltz, M.D., director of the Office of Drug Evaluation III in the FDA’s Center for Drug Evaluation and Research, said, "Moderate-to-severe plaque psoriasis can cause significant skin irritation and discomfort for patients, and today’s approval provides patients with another treatment option for their psoriasis."
"Patients and their health care providers should discuss the benefits and risks of brodalumab drug before considering treatment."
Brodalumab - Mechanism of Action
The active ingredient of the drug mainly acts by binding to a protein which causes inflammation and inhibits the inflammatory response which plays a role in plaque psoriasis.
Clinical Trial
The efficacy and safety of brodalumab drug were established in three randomized, placebo-controlled clinical trial on 4,373 participants who have moderate-severe plaque psoriasis who were eligible for systemic therapy or phototherapy.
Risk of Suicidal Ideation and Behavior
The patients who were treated with brodalumab drug in clinical trials had a risk of suicidal ideation and behavior. These users with a history of suicidality or depression may further have an increased risk of suicidal ideation or behavior.
The requirements of the Brodalumab Risk Evaluation and Mitigation Strategy (REMS) Program include
- Physicians who prescribe the drug must be certified with the program and must counsel patients about the risk. The patients have to be referred to a mental health professional if there are any new or worsening symptoms of depression or suicidality.
- A patient-prescriber agreement form has to be signed by the patient and they must be aware of the need for medical attention as they can experience new or worsening symptoms or changes in the mood.
- The pharmacies must also be certified and must dispense the drug to only authorized patients who receive brodalumab drug.
- Joint pain
- Headache
- Fatigue
- Diarrhea
- Throat pain
- Nausea
- Muscle Pain
- Influenza
- Low white blood cell count
- Fungal infections
Note: The drug must not be injected to patients who have active tuberculosis infections.
They must avoid immunizations with live vaccines when treated with brodalumab drug
Facts on Psoriasis
- Psoriasis occurs commonly in patients who are with a family history of the disease.
- People between the age of 15 and 35 years are more prone to psoriasis.
- The common form of psoriasis is plaque psoriasis where the patient skin is accompanied with redness and flaky silver-white scales.
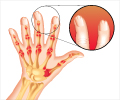
- Around 11% of people diagnosed with psoriasis are also being diagnosed with psoriatic arthritis.
- It is estimated that around 20,000 children below 10 years of age are diagnosed with psoriasis every year.
- About Psoriasis - (https://www.psoriasis.org/about-psoriasis)
- FDA approves new psoriasis drug - (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm)
Source-Medindia