Aegerion Pharmaceuticals' have come out with a novel drug used to treat a rare disease that causes extremely high levels of bad cholesterol. This drug has now received the nod by FDA.
Users of this drug must be cautious as there is a risk of liver damage, therefore the availability of this drug will be restricted. Moreover, the box will carry a ‘black-box ‘warning and this apparently is the FDA’s most strict safety warning.
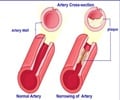
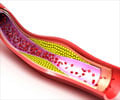
Nearly 3,000 people in America have been affected with this disease. Treatments like blood filtration have not been so effective and victims die prematurely by the age of 30 due to heart attacks or strokes.
Aegerion plans to start selling Juxtapid in January 2013. Shares of Aegerion Pharmaceuticals Inc., reached a new 52-week high of $25.74 and has gone up nearly 80 percent since October last year.
Source-Medindia