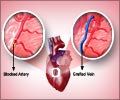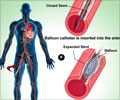
“The rigorous data from FDA clinical trials such as PROTECT I and PROTECT II demonstrate that complex, high-risk patients undergoing protected PCI with Impella 2.5 support experience reduced adverse events, improved quality of life and are able to return home faster with fewer repeat procedures,” said William O’Neill, M.D., Henry Ford Hospital in Detroit.
Impella is the only hemodynamic support device proven safe and effective for high risk PCI. This heart team approach has also been utilized for the treatment strategy for heart valve replacement. The clinical team can decide the duration of use though it is intended for up to six hours of use.
Source-Medindia