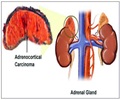
The committee also acknowledged that the drug led to hyperglycemia and elevations in liver enzymes in the patients but said that lack of other therapies for treating the condition means that the benefits outweighed the side effects. “In spite of significant concerns about hyperglycemia and uncontrolled diabetes, I think it's very highly probable that those glucose levels can be adequately controlled with available medications for managing diabetes, including insulin”, Brown University’s Robert Smith, who was part of the panel, said.
Source-Medindia