‘A range of factors other than just cost may influence the prescribing of TNF inhibitors for patients with rheumatoid arthritis.’
Tweet it Now
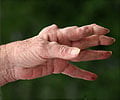
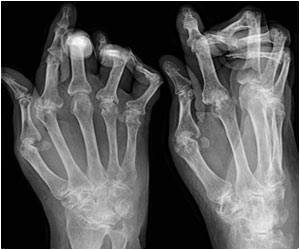
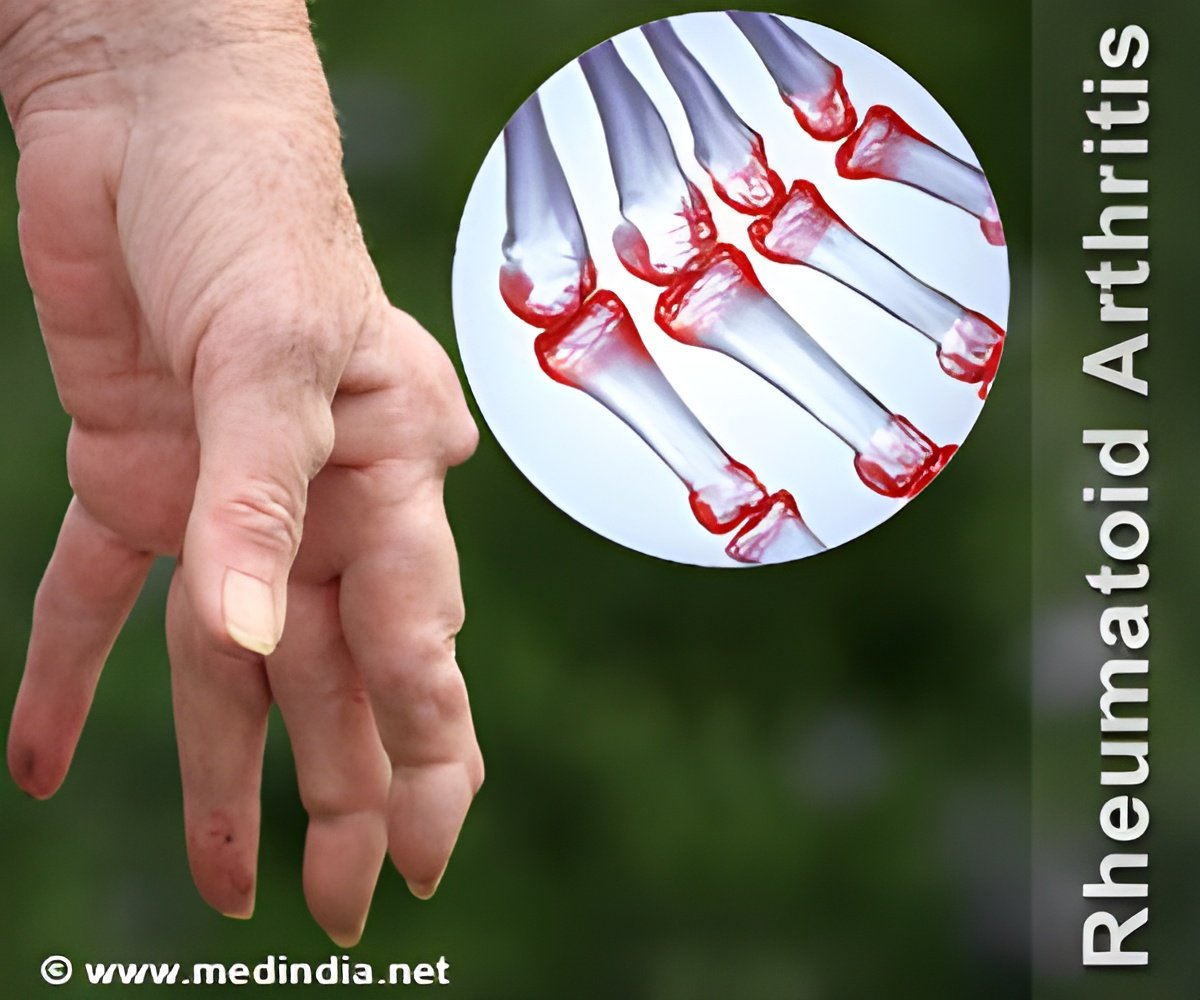
"Emergence of evidence, interpretation of clinical guidelines, patient involvement in decision making, desire for clinical autonomy and the involvement of clinical service commissioners have all been identified as influencing factors. We now need further research to explore whether these deviations from NICE guidance lead to differences in patient outcomes, or cost-effectiveness of care," Dr Gavan concluded." Currently, there are several different anti-TNF therapies recommended by NICE as options for treating RA. Treatment is usually in combination with methotrexate (assuming it is tolerated and not contraindicated), but only if there is evidence of severe disease (DAS28 disease activity score greater than 5.1), and the disease has not responded to intensive therapy with a combination of conventional disease modifying antirheumatic drugs (DMARDs).
When asked to discuss influences on key treatment decisions, a cross-section evaluation of UK consultant rheumatologists claimed that cost was rarely a factor to influence their choice of first-line anti-TNF, unless use of the least-expensive anti-TNF was imposed by local service commissioners. In contrast, cautious optimism was expressed towards using anti-TNF biosimilars first-line on the grounds of potential cost savings. Patient involvement in decision-making was perceived to be sacrificed in those units where using the cheapest anti-TNF was enforced.
Interpretation of NICE guidance varied, with some of the rheumatologists interviewed claiming it was too restrictive, and others seeing benefits in the flexibility it provides. Careful manipulation of the DAS28* disease activity score was cited by many of the interviewees as a way to maintain clinical autonomy and prescribe anti-TNF therapy if they believed it to be clinically appropriate in those RA patients whose disease didn't meet the NICE threshold.
Negotiated local exceptions to NICE guidance also facilitated clinical autonomy, with the use (and success) of individual funding requests for treatments varying between interviewees. Often advances in clinical evidence were used to justify deviations from guidelines. However, the influence of clinical evidence had a lesser role in dose-optimisation decisions in those RA patients in remission in whom evidence to guide such decisions is limited.
Advertisement