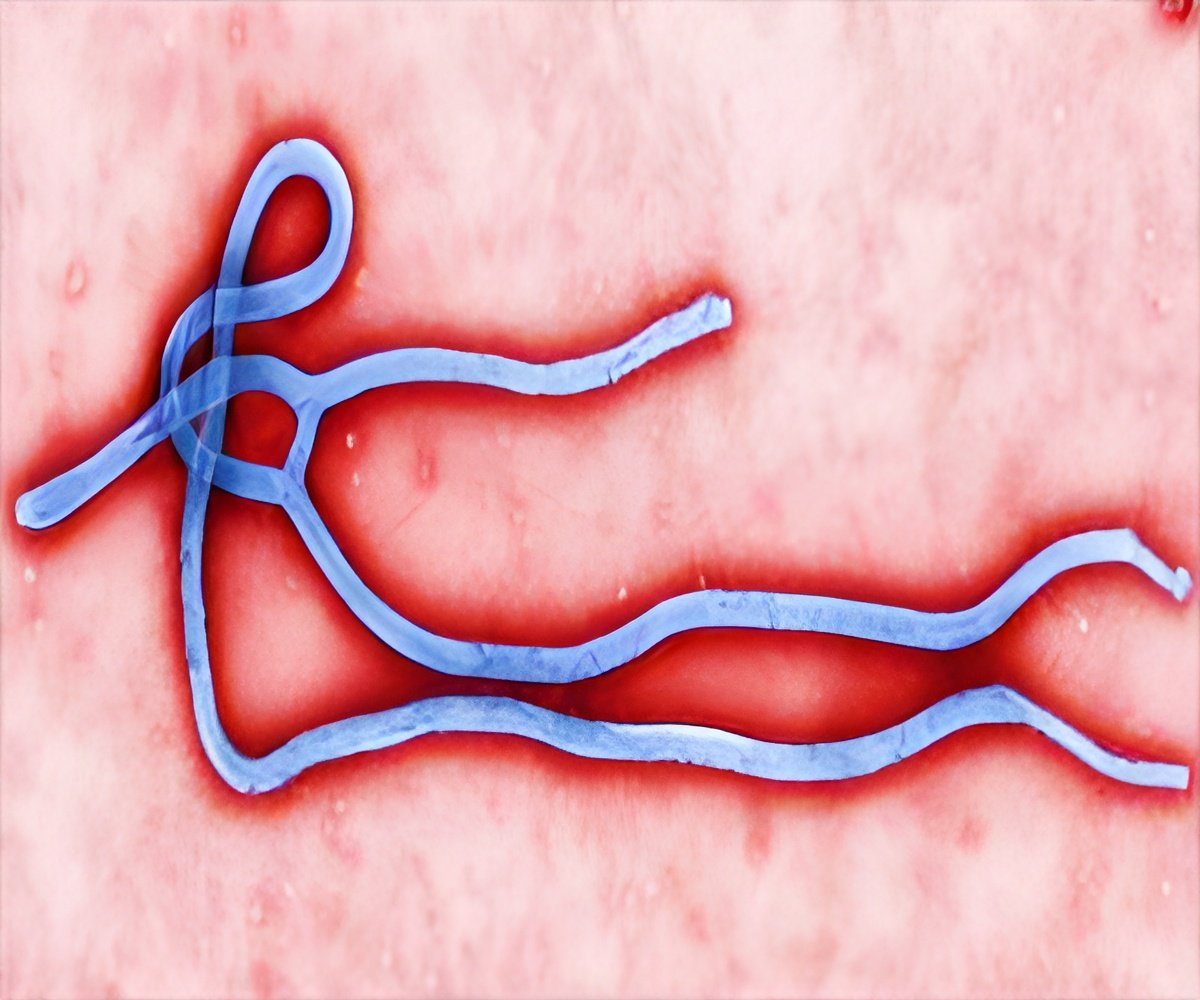
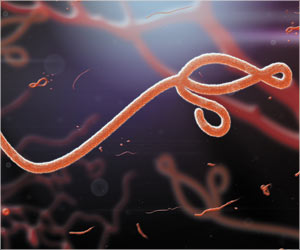
During the recent Ebola outbreak in West Africa, an experimental drug ZMapp was used to treat some of the healthcare workers infected with Ebola, even though the drug had never undergone a full-scale clinical trial. Not all those who were given the drug survived, but some who were cured of Ebola believed that it worked for them.
A clinical trial of ZMapp is going on in West Africa. The drug has been in the development phase for more than a decade. The US Food and Drug Administration has granted a fast track status to ZMapp, and this could speed its arrival on the market, reported the drug-maker.
Larry Zeitlin, president of LeafBio and Mapp Biopharmaceutical, said, "The decision by the FDA is an important milestone."
Prior to getting the fast-track status, ZMapp had been granted 'orphan drug' designation, which provides financial and other regulatory incentives meant to encourage development.
Kevin Whaley, chief executive officer of LeafBio and Mapp Biopharmaceutical, based in San Diego, California, said, "We are hopeful that this step will accelerate access to ZMapp once safety and efficacy are demonstrated to FDA's satisfaction in ongoing clinical trials."
Source-AFP