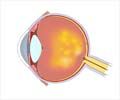
It is estimated that 250,000-300,000 patients in Europe alone suffer from this condition and surgery at an advanced stage is the major treatment option available currently. However, a single injection of Jetrea, the need for surgery could be avoided in many cases.
ThromboGenics holds the rights to market the drug in USA while its partner, Novartis would market the drug in Europe through its eye care division, Alcon.
Source-Medindia