Governments could also set up registers to trace implants, a measure that does not exist in every EU country.
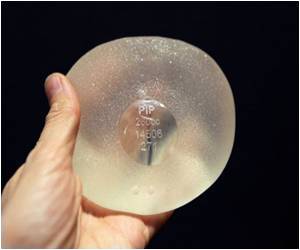
France vowed last week to strengthen the regulation and monitoring of prosthetics and called for EU-wide controls after several governments advised women to have implants made by French firm Poly Implant Prothese (PIP) removed.
"I am deeply concerned about the potential health impact for the many women, in Europe and in other parts of the world, who received faulty silicone breast implants manufactured by the French company PIP," said EU health commissioner John Dalli.
"The consequences for their health are still uncertain," he said, adding that he had ordered an in-depth study into the health impact of PIP implants.
Dalli has written to the EU's 27 health ministers urging them to take immediate action. He said the commission will present revisions to the medical devices legislation before the summer break.
Advertisement
Source-AFP