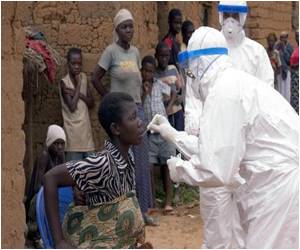
The clinical trial will be conducted in Sierra Leone’s Kambia district where maximum number of Ebola cases were registered. The treatment will be tested in an amalgamation of two vaccines.
AdVac dose is the first vaccine for prime patient’s immune system and the second vaccine, MVA-BN dose will be given two months later to boost their immune response, with the goal to strengthen the duration of their immunity.
"Never again can Ebola be allowed to cause the human suffering that the world has witnessed in West Africa and we remain committed as ever to helping the international community combat this disease”, said Dr Paul Stoffels, Chief Scientific Officer and Worldwide Chairman, Pharmaceuticals, JNJ.
Professor Peter Piot, Director of the London School of Hygiene & Tropical Medicine, which is one of the partners conducting the study, said, “The urgency of a vaccine that offers long-term protection of the population was needed, in order to prevent a rebirth of the virus.”
Currently there us no licensed vaccine to treat Ebola virus. The deadly disease outbreak began in March 2014 in the West African countries claiming more than 11,000 lives.
Advertisement