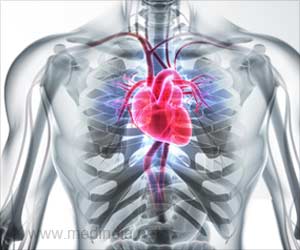
American scientists behind the phase I clinical trial of gene therapy for heart failure patients have announced some promising results, including improvements in several measures of the condition’s severity.
The multi-centre CUPID trial involved a small group of people to test a new treatment, with a view to evaluating its safety, determine a safe dosage range, and identify side effects.The patients enrolled for the study underwent a minimally invasive cardiac catheterization procedure that introduces a specially engineered gene, which stimulates production of an enzyme necessary for the heart to pump more efficiently.
NewYork-Presbyterian Hospital/Columbia University Medical Center was the first to offer the therapy in the New York City area.
The Phase I trial was initiated in May of 2007, and its results show that seven of nine patients who were given the drug showed improvements over six months in several areas: symptomatic (five patients), functional (four patients), biomarker (two patients) and left ventricular function/remodeling (six patients).
Two patients with pre-existing antibodies to the viral vector delivery system did not show improvements, according to the study report that was presented at the American Heart Association (AHA) Scientific Sessions 2008 in New Orleans.
The researchers said that the novel approach was found to have an acceptable safety profile, as determined by an independent safety committee.
Advertisement
The researchers are currently recruiting patients for the Phase II CUPID trial to further assess safety and effectiveness in patients with advanced heart failure.
Advertisement
Source-ANI
LIN