The new rules will cover every device, from the simplest plasters to life-support systems by way of in vitro diagnostic instruments, to ensure that they are correctly approved and then monitored in use.
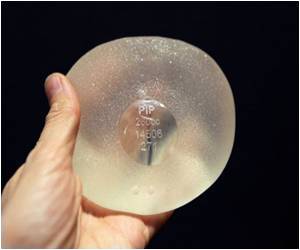
Earlier this year, the French government recommended that faulty breast implants which were prone to rupture made by Poly Implant Prothese (PIP) should be removed.
More than 400,000 women around the world are believed to have received PIP implants and many countries followed the French lead after the company was found to have used substandard, industrial-grade silicone gel.
"Everybody was shocked by the scandal ... (which) damaged the confidence of patients, consumers and healthcare professionals in the safety of the devices on which they rely every day," Dalli said.
Asked whether his regulations would have prevented the PIP scandal, he said they would "militate against such fraud" but the key issue was that once they were sold there had been no follow up to check the implants.
Advertisement
"If this had happened, the PIP scandal would have been detected many, many years before."
Advertisement
The aim is to give healthcare professionals better information on the benefits and risks while manufacturers will benefit from clearer rules, with those not complying excluded from the market.
The medical devices market in the 27 EU states plus Norway and Switzerland was worth 95 billion euros in 2009, the statement said.
The proposals now go to the European Council and the European Parliament for approval.
Source-AFP