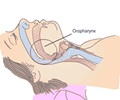
Modafinil tablets are generic versions of Provigil tablets manufactured by Cephalon Inc and are used in treating narcolepsy, shift work sleep disorders and excessive daytime sleepiness associated with obstructive sleep apnea. According to figures from IMS Health, modafinil tablets accounted for more than $1.2 billion in sales for the last 12 months ending March 2012.
Source-Medindia