The Public Citizen, a US advocacy group has demanded withdrawal of the Ortho-Evra contraceptive patch from the market, deeming it unsafe, far riskier than the pill.
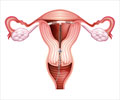
The birth control patch is a thin, beige, 1¾-inch (4½-centimeter) square patch that sticks to the skin. It releases hormones through the skin into the bloodstream to prevent pregnancy. Hormones are chemical substances that control the functioning of the body's organs.The combination of the hormones progesterone and estrogen in the patch prevents ovulation (the release of an egg from the ovaries during a girl's monthly cycle). If an egg isn't released, a girl can't get pregnant because there's nothing for a guy's sperm to fertilize.
The hormones in the patch also thicken the mucus produced in the cervix, making it difficult for sperm to enter and reach any eggs that may have been released. The hormones can also sometimes affect the lining of the uterus so that if the egg is fertilized it will have a hard time attaching to the wall of the uterus.
Ortho-Evra is a popular patch in the US, though demand has dropped over the years consequent on fears over its safety.
A 2005 investigation by news agency Associated Press found that patch users had higher rates of life-threatening blood clots than did women who took birth control pills.
Blood clots are a rare side effect for estrogen-related products. Some studies of the risk suggest that patch users have twice the risk of clots in the legs and lungs as do women who swallow the pill because patients absorb up to 60 percent more estrogen with the patch. The Food and Drug Administration (FDA) updated the patch’s label in 2005, 2006 and earlier this year with clot warnings.
Advertisement
Besides two previously unpublished studies from Johnson & Johnson researchers found higher estrogen exposure from the patch even before it won federal approval in 2001.
Advertisement
The drug agency said it had not had an opportunity to review the petition by Public Citizen.
Source-Medindia
GPL/L