At this point, the data that FDA has reviewed have not shown a clear connection between bisphosphonate use and a risk of atypical subtrochanteric femur fractures, the agency said and added it was working closely with outside experts, including members of the recently convened American Society of Bone and Mineral Research Subtrochanteric Femoral Fracture Task Force, to gather additional information that may provide more insight into this issue.
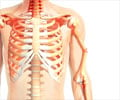
Based on published case reports of atypical subtrochanteric femur fractures occurring in women with osteoporosis using bisphosphonates, FDA, in June 2008, requested information from all bisphosphonate drug manufacturers regarding this potential safety signal. All available case reports and clinical trial data were requested. FDA's review of these data did not show an increase in this risk in women using these medications.
In addition, FDA reviewed a December 2008 article in the Journal of Bone and Mineral Research by Abrahamsen et al1, that analyzed data from two large observational studies in patients with osteoporosis. The authors concluded that atypical subtrochanteric femur fractures had many similar features in common with classical osteoporotic hip fractures, including patient age, gender, and trauma mechanism. The data showed that patients taking bisphosphonates and those not taking bisphosphonates had similar numbers of atypical subtrochanteric femur fractures relative to classical osteoporotic hip fractures.
“This communication is in keeping with FDA's commitment to inform the public about its ongoing safety review of drugs. The agency will continue to review new information as it becomes available and will update the public once the agency's review is complete,” the agency said.
Advertisement
GPL