"What we have found is HspB1 is a protective mechanism that tries to get rid of the toxic oligomers or aggregates of amyloid beta that occur in Alzheimer's," Dr. Anil G. Cashikar, Biochemist at Georgia Health Sciences University's Center for Molecular Chaperones and Radiobiology, said.
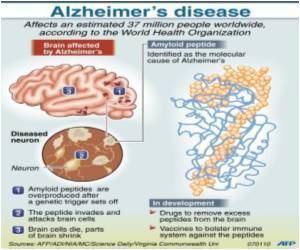
Amyloid beta peptide, or Abeta, is believed to start the cascade of events that leads to brain cell damage and death in Alzheimer's: as levels increase, the peptide starts clumping in the brain. In fact, high levels in the spinal fluid are a diagnostic marker for the disease.
Molecular chaperones are known for their propensity to respond to disease-producing misfolded proteins, which is how the body views excessive Abeta.
While resulting plaques occupy prime real estate in the brain, it's still better than toxic Abeta killing neurons, Cashikar said.
"We think maybe the system gets overwhelmed," he stated.
Advertisement
The new study also showed that neurons from HspB1-deficient mice were more sensitive to the toxic ravages of Abeta.
Advertisement
He wants to exploit this natural system by developing a smaller version of the molecular chaperone that could be put into the bloodstream to leach excess Abeta from the brain.
The brain has a natural protective mechanism that likely would prevent its direct application. However, the natural affinity of amyloid beta and HspB1 indicates a more distant approach could be effective.
"We want to come up with smaller versions of HspB1 that can be put into the bloodstream so you can sop up the material from the brain into the blood where it can be cleared more efficiently," he said.
The study has been published in Molecular and Cellular Biology.
Source-ANI













