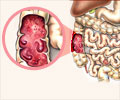
Clinical data presented today at the annual meeting of the American College of Gastroenterology (ACG) illustrates that REMICADE significantly reduces the incidence of colectomy surgeries for patients with moderately to severely active ulcerative colitis (UC). According to a primary analysis of long-term extension data from the Active Ulcerative Colitis 1 and 2 trials (ACT trials), there is a 41 percent reduction in the incidence of colectomy, the surgical removal of the colon, in patients receiving REMICADE through 54 weeks, compared to those receiving placebo (p=0.015). REMICADE is the first and only biologic approved for the treatment of ulcerative colitis.
The analysis of the ACT extension trials included 728 patients from the initial ACT 1 and 2 trials, of whom 630 (86 percent) had complete follow-up through 54 weeks to ascertain whether they underwent colectomy surgery."These data illustrate that treatment with REMICADE significantly reduces the need for life-altering colectomy in patients with refractory ulcerative colitis," said Paul Rutgeerts, M.D., Ph.D., University Hospital Gasthuisberg, University of Leuven; Leuven, Belgium. "REMICADE may offer patients who fail other therapies the possibility of avoiding costly surgeries and hospitalizations while managing the symptoms of this debilitating disease long-term."
Separate data also presented at ACG today show the significant impact of colectomy on ulcerative colitis patients' use of healthcare services, including inpatient and outpatient hospital procedures, emergency room and physician visits, and laboratory and drug costs. A retrospective analysis of 411 patients, identified from the U.S. PharMetrics database as having UC and a colectomy between January 1, 2000, and June 30, 2005, found that total utilization of healthcare services increased significantly in the 12 months following colectomy, including inpatient and outpatient procedures (P < 0.05).
"These diseases are devastating to patients and their loved ones and we are excited to see more and better therapies become available to ease their suffering," said Richard J. Geswell, President, Crohn's & Colitis Foundation of America.
Patients with moderate to severe UC, defined as a baseline Mayo score ¡Ý 6 and ¡Ü 12, who were unresponsive to or intolerant to at least one standard therapy, including corticosteroids, immunosuppressants or 5ASAs, were enrolled in ACT 1 (n=364) or ACT 2 (n=364). The 728 patients were randomized to receive REMICADE 5 mg/kg, REMICADE 10 mg/kg or placebo at weeks 0, 2, 6 and every subsequent 8 weeks through week 22 (ACT 2) or week 46 (ACT 1). Patients were allowed to continue to receive conventional therapy.
Patients who completed treatment through the final infusion visit and who, in the opinion of the investigator, could benefit from continued treatment with REMICADE could enroll in the extension trials. Across multiple sites, one hundred and eighteen (118) patients were entered into the ACT 1 extension and 111 patients were entered into the ACT 2 extension.
Overall, REMICADE was generally well tolerated in the long-term extensions, with less than five percent of patients discontinuing therapy due to an adverse event (AE). As previously reported, other notable serious adverse events (SAEs) included: prostate cancer, breast cancer, pneumonia, sarcoidosis, abscess and a death following Histoplasmosis pneumonia. (See Important Safety Information below).
A total of 411 patients were included in the analysis, and researchers collected data for each patient both 12 months prior to the colectomy and 12 months following colectomy. The researchers focused on the levels of inpatient, outpatient, emergency room, physician, laboratory and drug costs of care and found that healthcare utilization increased during the 12 months following colectomy. The increases were primarily driven by inpatient and outpatient visits.
The data showed that inpatient encounters increased during the 12 months after colectomy, compared with the number of encounters during the 12 months before colectomy (1.8 vs. 0.9). There were also increases in outpatient visits (19.4 vs. 8.7), emergency room visits (0.9 vs. 0.8), physician visits (14.9 vs. 13.9) and laboratory visits (2.2 vs. 2.1) during the post-colectomy period, compared with the pre-colectomy period.
UC is a chronic inflammatory bowel disease affecting nearly 500,000 people in the U.S. The disease is characterized by inflammation and ulceration of the colonic mucosa, or innermost lining of the intestine, which causes bloody stools and severe diarrhea. Tiny open sores, or ulcers, form on the surface of the intestinal lining where they bleed and produce pus and mucus. Because the inflammation makes the colon empty frequently, symptoms often lead to unwanted weight loss, blood loss and a host of secondary complications. The cause of UC is not known and there is no cure.
REMICADE is the global market leader among anti-tumor necrosis factor alpha (TNF-alpha) therapies and is the only anti-TNF-alpha treatment approved in three different therapeutic areas: gastroenterology, rheumatology and dermatology. REMICADE has demonstrated broad clinical utility in Crohn's disease (CD), rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriatic arthritis (PsA), ulcerative colitis (UC), pediatric Crohn’s disease (PCD) and psoriasis (PsO). The safety and efficacy of REMICADE have been well established in clinical trials over the past 14 years and with more than 925,000 patients treated worldwide through commercial experience.
REMICADE, in combination with methotrexate, is indicated for reducing signs and symptoms, inhibiting the progression of structural damage and improving physical function in patients with moderately to severely active RA. REMICADE is the only biologic indicated for reducing signs and symptoms and inducing and maintaining clinical remission in adult and pediatric patients with moderately to severely active CD who have had an inadequate response to conventional therapy. REMICADE is also indicated for reducing the number of draining enterocutaneous and rectovaginal fistulas and maintaining fistula closure in adult patients with fistulizing CD. In December 2004, REMICADE was approved for reducing signs and symptoms in patients with active AS. In May 2005, REMICADE was approved for reducing signs and symptoms of active arthritis in patients with PsA. Additionally, in September 2005, REMICADE was approved for reducing signs and symptoms, achieving clinical remission and mucosal healing, and eliminating corticosteroid use in patients with moderately to severely active UC who have had an inadequate response to conventional therapy. This approval makes REMICADE the first and only biologic approved for the treatment of moderate to severe UC. In addition, on May 19, 2006, REMICADE was approved for reducing signs and symptoms and inducing and maintaining clinical remission in pediatric patients with moderately to severely active CD who have had an inadequate response to conventional therapy. This approval establishes REMICADE as the first and only biologic therapy approved for the treatment of pediatric CD. In August 2006, REMICADE received the expanded indication for inhibiting the progression of structural damage and improving physical function in patients with psoriatic arthritis. In September 2006, REMICADE was approved for the treatment of adult patients with chronic severe plaque psoriasis. In October 2006, REMICADE was approved for maintaining clinical remission and mucosal healing in patients with moderately to severely active UC, who have had an inadequate response to conventional therapy.
REMICADE is unique among available anti-TNF biologic therapies. Unlike self-administered therapies that require patients to inject themselves frequently, REMICADE is the only anti-TNF biologic administered directly by caregivers in the clinic or office setting. In RA (3 mg/kg), CD (5 mg/kg), PsA (5 mg/kg), UC (5 mg/kg), PCD (5 mg/kg), and PsO (5 mg/kg), REMICADE is a two-hour infusion administered every 8 weeks, following a standard induction regimen that requires treatment at weeks 0, 2 and 6. As a result, REMICADE patients may require as few as six treatments each year. In AS (5 mg/kg), REMICADE is a two-hour infusion administered every 6 weeks, following a standard induction regimen that requires treatment at weeks 0, 2 and 6.
Source-Eurekalert
GAN/V