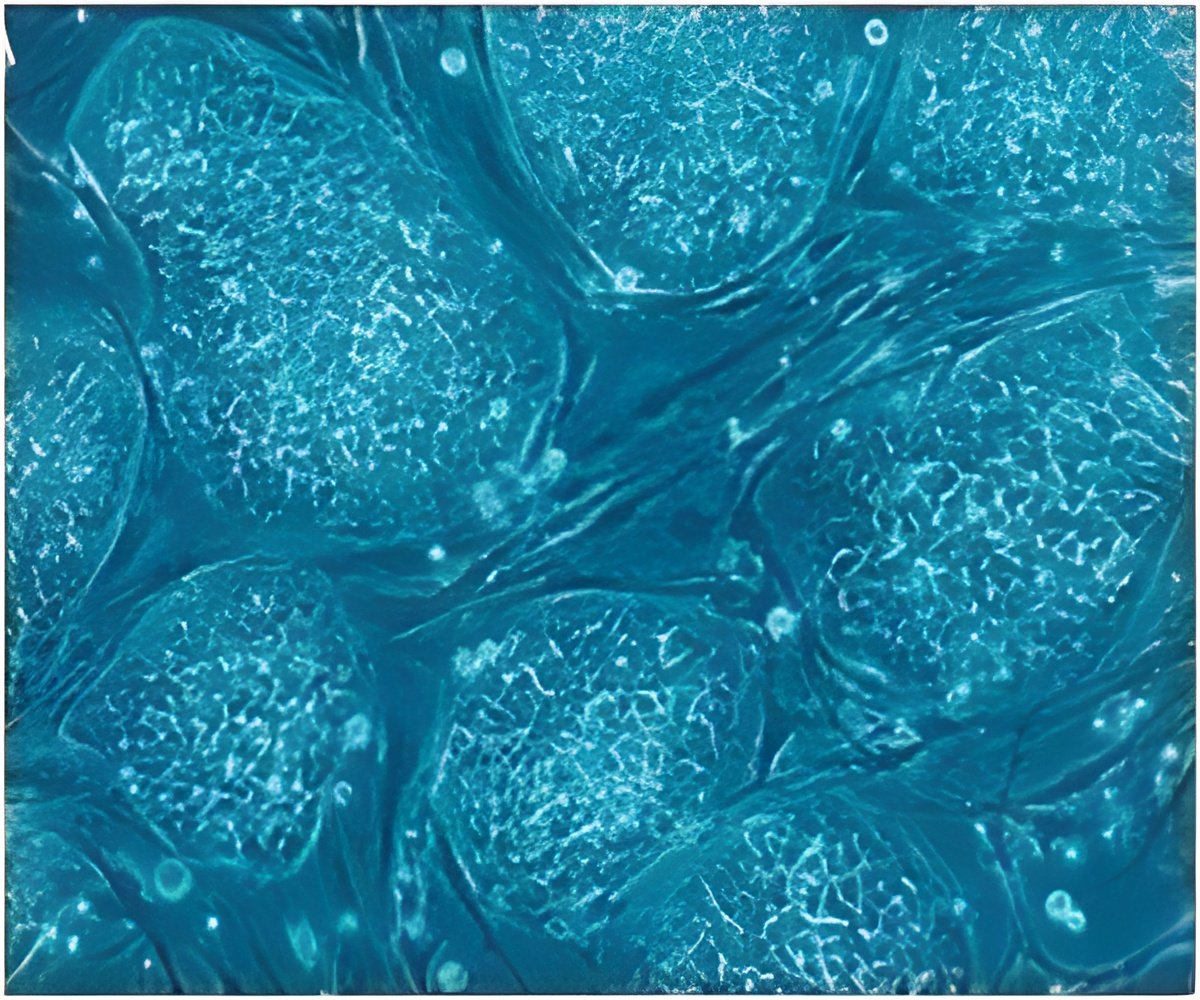
The US FDA has now approved the first cord-blood transplant therapy- HEMACORD. Cord blood transplants are used for the treatment of blood cancer and certain inherited metabolic or immune system disorders.
HEMACORD contains hematopoietic progenitor cells (HPCs) derived from human cord blood. When these HPCs are infused in the body, the cells transit to the bone marrow where they undergo division and maturation. As they circulate in the blood stream they can help build new blood cells or resurrect their working including immune function.
The cord blood treatment comprises a boxed warning related to risks of Graft Versus Host Disease(GVHD), engraftment syndrome, graft failure and infusion effects.
This could also turn out to be fatal and therefore patients on HEMACORD should be inspected minutely.
HEMACORD is manufactured by the New York Blood Center (NYBC).
Source-Medindia