Medical devices manufacturer, Cook Medical has revealed that it has received a 510(k) clearance by the Food & Drug Administration (FDA) for its gastrointestinal stent to be used in treating GI obstructions caused by cancer.
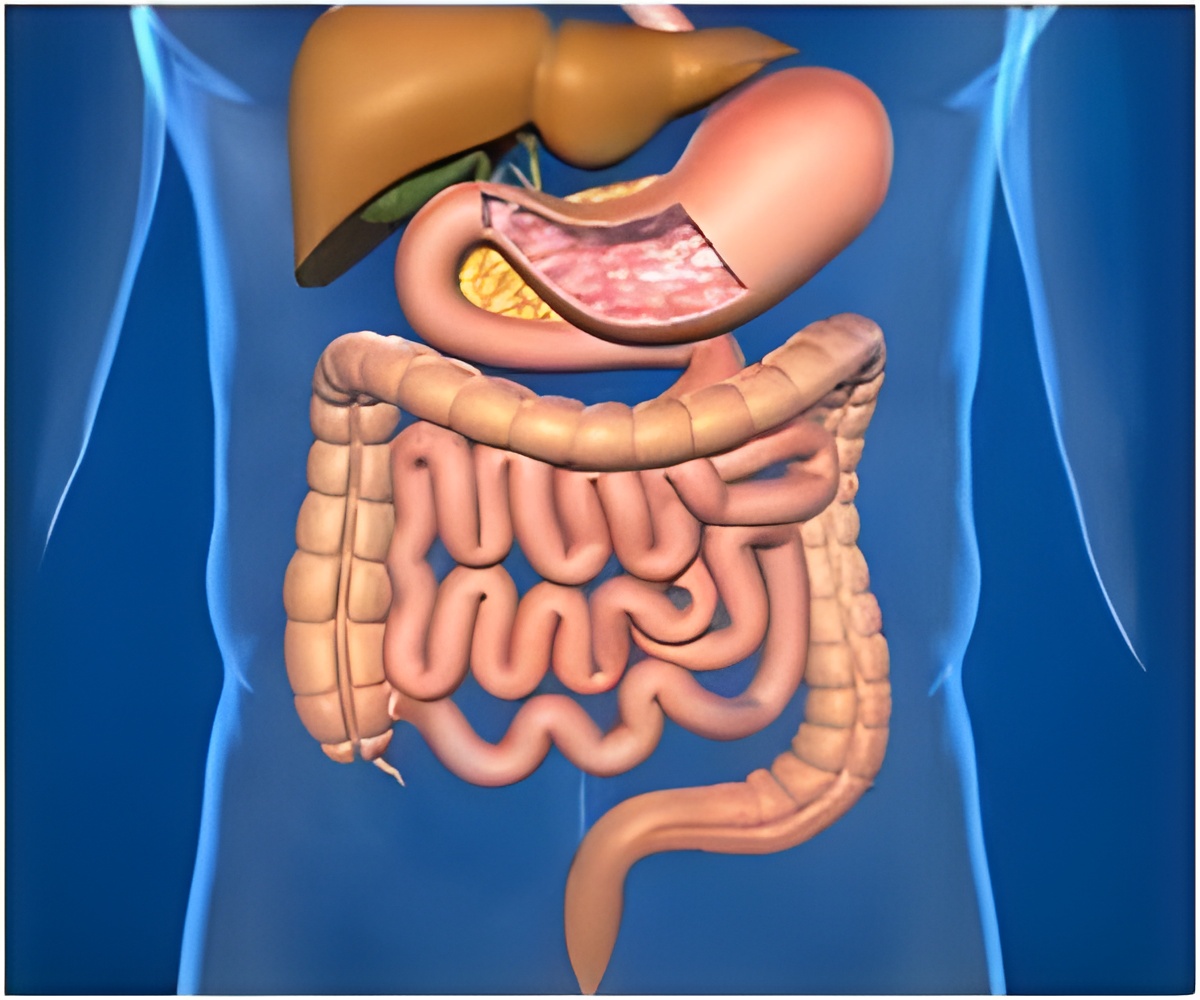
The company said that its Evolution Duodenal Controlled-Release Stent will help provide a more precise treatment for gastric outlet obstruction (GOO),compared to the currently available solutions for treating the same. It added that the new stent would assume the shape of to the patient’s duodenum to reduce the risk of migration.
Gastric outlet obstruction (GOO) is a common complication in GI-related cancers and manifests as abdominal pain, vomiting and constipation. Bypass surgery is used to treat these obstructions, but stents are a better option.
Cook’s stent will compete with other available stents, but the main advantage is that it has better flexibility and can also reduce the risk of perforation of the intestines.
Source-Medindia