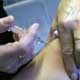
By July, China may manufacture an A(H1N1)influenza vaccine for human use.
Yin Hongzhang, head of the biology production department under the State Food and Drug Administration (SFDA), says that studies on samples of a flu strain that China is likely to receive by early June may make it possible."The country has set up a green channel between the World Health Organization (WHO) and the Chinese drug makers. As soon as the WHO releases the vaccine strain, drug companies will be informed and will start manufacturing as soon as they can," Xinhua quoted Yin as saying.
Given that vaccine manufacturing requires high-level safety, Yin says that China must produce one in line with WHO protocols.
WHO last week said that drug companies wouldn't be able to make an A(H1N1) vaccine until mid-July at the earliest as the virus was growing slowly in labs, making it difficult for scientists to get the key ingredient for a vaccine.
According to the China Daily, Yin noted that WHO was not sure yet whether the A(H1N1) flu should be categorized as seasonal or pandemic, which was a problem for drug makers.
Yin also revealed that 11 drug companies in China could produce seasonal flu vaccines, but only one could make pandemic flu vaccines.
Advertisement
He further said that China would become the first country to guarantee vaccine supplies for medical staff if the A(H1N1) influenza was confirmed as pandemic.
Advertisement
It would take another 45 days to two months to take full effect, which would normally last for more than one year, Yin added.
Source-ANI
TAN