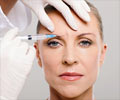
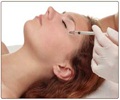
Health Canada, the federal agency, has announced it is reviewing the safety of the wrinkle treatment Botox and of a similar drug called Myobloc.
The move comes only days after a call from a U.S. lobby to increase the warnings on Botox.Last week, Dr. Sidney Wolfe of Public Citizen Health Research Group said severe reactions including deaths were linked to Botox.
Both Botox and Myobloc use botulinum toxin, which blocks nerve impulses to muscles, causing them to relax. But in a few cases, the toxin had spread to other parts of the body, resulting in problems including paralysis of respiratory muscles and difficulty swallowing, potentially leading to food or liquids entering the lungs and causing aspiration pneumonia, Wolfe had said.
Botox is traditionally used as a cosmetic treatment to ease wrinkles, but is also used for treating rigid neck muscles, a condition known as cervical dystonia. Myobloc is approved in Canada for treating cervical dystonia.
The company that makes Botox disputed claims that complications from Botox cosmetic treatments had resulted in serious problems.
Allergan Canada said in a news release issued Wednesday that in the 18 years since the product was first approved worldwide, 'reports of serious adverse events following Botox injection have been rare and there has never been a single reported death where a causal link to Botox Cosmetic was established.'
Advertisement
Canadian officials say that if the review identifies any new safety information, it will be made public.
Source-Medindia
GPL/K