Only last month the US Food and Drug Administration (FDA) panel had suggested that aprotinin, sold by Bayer AG under brand name Trasylol, be allowed to remain in market despite reports of increased risk of death and other serious side effect.
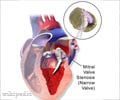
But manufacturers themselves announced Thursday that a large Canadian-led trial of the drug had been halted over concerns that the drug could increase the risk of death among people who receive it.The injectable drug is used to prevent excessive blood loss during heart surgery brought on by the blood thinner heparin and mechanical tubing used in the heart-bypass machine.
The aim of the trial, known by its acronym BART, is to compare the effectiveness and safety of aprotinin, aminocaproic acid and tranexamic acid — three different kinds of antifibrinolytic drugs.
Approximately 3,000 high-risk cardiac surgery patients undergoing either a re-operation for a coronary heart bypass graft or aortic valve replacement, or combined valve or valve-graft procedures were to be randomly assigned to one of the drugs.
When a periodic analysis of data from the trial showed increased deaths among the aprotinin group, the trial's executive board decided to cut off enrolment in the study and informed drug regulators and the company.
Following Bayer’s announcement the U.S. Food and Drug Administration said the agency would consider the new evidence as part of its ongoing deliberations on whether the drug should be removed from the market or have additional warnings on its label.
Advertisement
"In light of the preliminary BART study findings, FDA anticipates re-evaluation of the overall risks and benefits of Trasylol. …" the U.S. drug regulator said. "Until this process has been completed, health-care providers who are considering use of Trasylol should be aware of the risks and benefits described in the labelling for Trasylol and the accumulating data suggesting Trasylol administration increases the risk for death compared to other antifibrinolytic drugs."
Advertisement
"Bayer will continue to work closely with medical experts, the FDA and health authorities in countries where Trasylol is marketed, to re-evaluate the overall risk-benefit of the product and will evaluate the need for a label change and/or other actions as additional data and analyses become available from the BART trial," the company said in a statement.
Source-Medindia
GPL/S