"Treatment for anal cancer is challenging; the use of Gardasil as a method of prevention is important as it may result in fewer diagnoses and the subsequent surgery, radiation or chemotherapy that individuals need to endure," said Karen Midthun, director of the FDA Center for Biologics Evaluation and Research.
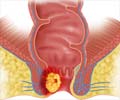
Although anal cancer is rare in the general population, its occurence is increasing especially among gay men, officials noted.
The American Cancer Society estimates about 5,300 people are diagnosed with anal cancer each year in the United States, with more women diagnosed than men.
Source-AFP